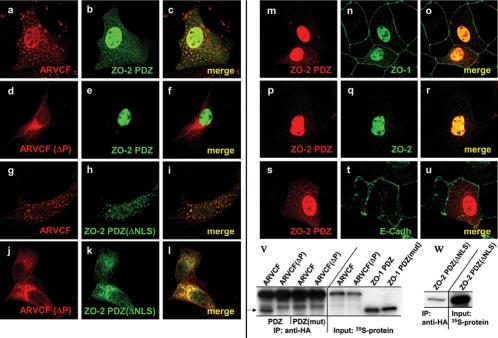
Figure 8.
Role of ZO-2 in nuclear localization of ARVCF. MDCK cells expressing ARVCF (a-c and g-i) or ARVCF(ΔP) (d-f and j-l) were transfected with cDNAs encoding the ZO-2 PDZ domains (a-f) or ZO-2 PDZ domains with an inactivated NLS [ZO-2 PDZ(ΔNLS), g-l] and analyzed by immunofluorescence staining and confocal microscopy. Colocalization of the respective proteins is yellow in the merged images. In m-u, the distribution of endogenous ZO-1, ZO-2, and E-cadherin was analyzed in MDCK cells transfected with a plasmid encoding the ZO-2 PDZ domains. ZO-2 constructs carried an N-terminal Xpress tag. Colocalization of the respective proteins is yellow in the merged images. v, mutation of the PDZ domains in ZO-1 PDZ abolishes the interaction with ARVCF. ARVCF or ARVCF(ΔP) and ZO-1 PDZ or ZO-1 PDZ(mut) were in vitro cotranslated in the presence of [35S]methionine. ARVCF or ARVCF(ΔP) was then immunoprecipitated, the precipitates fractionated by SDS-PAGE, and the dried gels used for autoradiography. w, deletion of the NLS in ZO-2 PDZ(ΔNLS) does not affect the interaction with ARVCF. ARVCF and ZO-2 PDZ(ΔNLS) were in vitro cotranslated in the presence of [35S]methionine, ARVCF was then immunoprecipitated, the precipitates fractionated by SDS-PAGE, and the dried gels used for autoradiography. An aliquot of the in vitro translation reaction was directly analyzed to monitor the amount of protein added to the reaction. The arrow shows the location of ZO-1 PDZ (v).