Abstract
Purpose
The prognosis of HIV-infected patients with non-Hodgkin lymphoma in the highly active antiretroviral therapy (HAART) era approaches that of the general population when they are treated with the same protocols. We analyzed the outcome of patients with Hodgkin lymphoma (HL) treated with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in the HAART era according to HIV serostatus to establish whether this also holds true for HL.
Patients and Methods
From 1997 to 2010, 224 patients newly diagnosed with HL, of whom 93 were HIV positive, were consecutively treated with ABVD chemotherapy. HIV-positive patients had more high-risk disease according to the International Prognostic Score (IPS) than HIV-negative patients (IPS ≥ 3: 68% v 26%, respectively; P < .001). Forty-seven HIV-positive patients had a CD4 count less than 200/μL, and 92 patients received HAART during chemotherapy.
Results
The complete response rate was 74% for HIV-positive patients and 79% for HIV-negative patients (P = not significant). After a median follow-up of 60 months (range, 8 to 174 months), 23 patients (16 HIV-negative and seven HIV-positive patients) have experienced relapse at a median time of 6 months (range, 1 to 106 months). Five-year event-free survival (EFS) was 59% (95% CI, 47% to 70%) for HIV-positive patients and 66% (95% CI, 57% to 74%) for HIV-negative patients (P = not significant). Five-year overall survival (OS) was 81% (95% CI, 69% to 89%) and 88% (95% CI, 80% to 93%) for HIV-positive and HIV-negative patients, respectively (P = not significant). HIV status did not predict OS or EFS on multivariate analysis including IPS and HIV status.
Conclusion
This mature study demonstrates that HIV-positive patients with HL have more extensive disease with more adverse prognostic factors than HIV-negative patients, but when treated with ABVD, HIV infection does not adversely affect OS or EFS.
INTRODUCTION
The incidence of both non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) is significantly increased in people living with HIV infection,1,2 partly related to their low CD4 cell count. The introduction of highly active antiretroviral therapy (HAART) has resulted in a significant reduction in the incidence of some types of NHL, notably primary CNS lymphoma, which used to present in patients with extremely low CD4 counts.3 In contrast, there is no clear agreement regarding the effect of HAART on the incidence of HL, with some series reporting an increased incidence4,5 and others reporting no changes.6–8 Notwithstanding the impact of HAART on the incidence of lymphoma, malignancy accounts for one third of deaths in patients living with HIV, and lymphoma is the most common cause of malignancy-related death in this population.9 In addition, HAART has resulted in a significant improvement in the prognosis of patients with HIV and NHL. Several studies have demonstrated that the outcome of patients with HIV infection and diffuse large B-cell lymphoma10 or Burkitt's lymphoma11 is comparable to that of HIV-negative patients treated with the same chemotherapy regimens. There has not been a similar analysis in patients with HL. The aim of this retrospective study was to analyze the outcome of patients diagnosed with HL and treated with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy in the HAART era according to their HIV status.
PATIENTS AND METHODS
Study Population
This retrospective study included patients diagnosed with classical HL (cHL) and treated with ABVD chemotherapy during the HAART era. The HAART era was defined as starting in January 1997, when HAART became widely available in the United Kingdom. From 1997 to 2010, 224 patients (including 93 patients with HIV infection) were newly diagnosed with cHL and consecutively treated with ABVD chemotherapy in five university hospitals in London. The cohort comprised 131 HIV-negative patients from St Bartholomew's Hospital and 93 HIV-positive patients from St Bartholomew's Hospital (n = 20), Chelsea & Westminster (n = 45), Royal Free Hospital (n = 15), King's College Hospital (n = 9), and Guy's and St Thomas Hospital (n = 4). There were no statistically significant differences in the clinical characteristics among patients in different centers (International Prognostic Score [IPS] ≥ 3: St Bartholomew's Hospital, 58%; Chelsea & Westminster, 73%; Royal Free Hospital, 67%; King's College Hospital, 67%; Guy's and St Thomas Hospital, 75%; P = .8). The management of patients (in terms of initial investigations, treatment for early- and advanced-stage disease, the role of radiotherapy, and administration of HAART and opportunistic infection prophylaxis in HIV-positive patients) was uniform across centers. There were no significant differences in disease-free survival (DFS), overall survival (OS), or event-free survival (EFS) in HIV-positive patients according to center. Patient characteristics are listed in Table 1. During the same period of time, 13 further HIV-seropositive patients were diagnosed with cHL and did not receive ABVD for the following reasons: localized disease treated with local radiotherapy (n = 1); treatment with other regimens (n = 7); death as a result of progression before starting treatment (n = 4); and unrelated death before starting treatment (n = 1). Similarly, during the study period, 179 HIV-negative patients were diagnosed with cHL and 48 (27%) were treated with regimens other than ABVD either because of the presence of localized disease treated with local radiotherapy (six patients) or because of treatment with alternative chemotherapy regimens (42 patients). The latter included treatment with a nonanthracycline regimen in 14 elderly or frail patients (8%) or treatment in the setting of a clinical trial.
Table 1.
Patient Demographics and Clinical Characteristics According to HIV Status
| Demographic or Clinical Characteristic | HIV-Positive Patients (n = 93) |
HIV-Negative Patients (n = 131) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Male | 83 | 89 | 75 | 57 | < .001 |
| Age, years | |||||
| Median | 41 | 31 | |||
| Range | 26-73 | 16-70 | |||
| ≥ 45 | 31 | 33 | 26 | 20 | .03 |
| Histologic subtype | < .001 | ||||
| Nodular sclerosis | 15 | 16 | 100 | 76 | |
| Mixed cellularity | 51 | 55 | 27 | 21 | |
| Lymphocyte depleted | 3 | 3 | 1 | 1 | |
| Unknown | 24 | 26 | 3 | 2 | |
| “B” symptoms | 75 | 81 | 52 | 40 | < .001 |
| WBC, × 109/L | |||||
| Median | 4.3 | 9.5 | |||
| Range | 0.3-14.1 | 2-39 | |||
| ≥ 15 | 0 | 0 | 22 | 17 | < .001 |
| Lymphocyte count, × 109/L | |||||
| Median | 0.9 | 1.5 | |||
| Range | 0.09-3.1 | 0.1-3.9 | |||
| < 0.6 | 64 | 68 | 6 | 5 | < .001 |
| Hemoglobin, g/dL | |||||
| Median | 10.9 | 12.2 | |||
| Range | 4.1-15.3 | 2.2-16.6 | |||
| < 10.5 | 41 | 44 | 27 | 21 | < .001 |
| Albumin, g/L | |||||
| Median | 33 | 42 | |||
| Range | 10-49 | 21-54 | |||
| < 40 | 73 | 79 | 48 | 37 | < .001 |
| Bone marrow involvement | 42 | 45 | 5 | 4 | < .001 |
| Spleen involvement | 23 | 25 | 7 | 5 | < .001 |
| Lung involvement | 5 | 5 | 11 | 8 | .4 |
| Liver involvement | 17 | 18 | 15 | 11 | .2 |
| Stage | < .001 | ||||
| I | 6 | 6 | 14 | 11 | |
| II | 13 | 14 | 72 | 55 | |
| III | 24 | 26 | 18 | 14 | |
| IV | 50 | 54 | 27 | 21 | |
| International Prognostic Score | < .001 | ||||
| 0-2 | 29 | 31 | 92 | 70 | |
| 3-7 | 63 | 68 | 34 | 26 | |
| Unknown | 1 | 5 | 4 | ||
Characteristics and Management of Patients With HIV Infection
Nine patients (10%) were concomitantly diagnosed with HIV infection and cHL, whereas 22 patients (24%) had a previous diagnosis of AIDS. The median CD4 cell count at diagnosis of cHL was 185/μL (range, 4 to 1,160/μL), and 52% of patients had a CD4 count of less than 200/μL. Among 87 patients with available data, 52 patients (60%) had an undetectable plasma HIV viral load at the time of cHL diagnosis. The median viral load for the remainder of patients was 3,060 copies/mL (range, 52 to 7,490,420 copies/mL). Seventy-three patients were receiving HAART before the diagnosis of cHL (either because of an AIDS-defining illness or because of a low CD4 count), and 92 patients received HAART concomitantly during chemotherapy (no data were available for the remaining patient). HAART was defined as a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor along with a backbone of at least two nucleoside reverse transcriptase inhibitors, as per British HIV Association guidelines.12 When possible, ritonavir boosted protease inhibitors were avoided because of the reported increased risk of myelotoxicity.13 All patients received opportunistic infection prophylaxis (fluconazole for candidiasis, acyclovir for herpes simplex virus, trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis jiroveci, and azithromycin for Mycobacterium avium).
Diagnosis and Staging
Histologic diagnosis was based on local review according to WHO criteria.14 All patients underwent standard staging procedures including a neck-chest-abdomen-pelvis computed tomography scan. A bone marrow (BM) biopsy was performed in all patients with HIV infection and in HIV-negative patients with advanced-stage disease (“B” symptoms, > four areas, bulky disease, stage III or IV) or with mixed cellularity subtype.
Chemotherapy Protocol
ABVD chemotherapy was administered every 28 days, as previously described.15 Patients with early-stage disease (stage I to IIA with no bulky disease or other adverse prognostic factors) received four cycles of ABVD followed by involved-field radiotherapy (IFRT), whereas the remainder of patients received six cycles of ABVD with IFRT to areas of bulky disease or residual masses. Thus, 28 patients (seven HIV-positive patients, including one with bulky disease and four with B symptoms undertreated as a result of HIV infection) received treatment for early-stage HL with four courses of ABVD plus IFRT, whereas 195 patients (85 HIV-positive patients, among whom two who were overtreated as a result of HIV infection) received treatment for advanced-stage HL with six courses of ABVD. Granulocyte colony-stimulating factors were administered according to oncology guidelines as secondary prophylaxis.
Definitions
Response was assessed after completing treatment (ie, after IFRT, for patients who received IFRT) using standard response criteria in use at the time.16 Response rate included complete response (CR) and CR uncertain (CRu). OS was defined as the time from HL diagnosis to death from any cause, with surviving patients censored at last follow-up. EFS was defined for all patients as time from diagnosis to failure of treatment (including not achieving CR/CRu or relapse after CR/CRu) or death from any cause.17 DFS was defined for patients achieving CR/CRu as the time from response to disease recurrence or death as a result of HL or acute toxicity of treatment.17
Statistical Analysis
All patients with available data were included in the main analysis. Differences between HIV-positive and HIV-negative patients in the distribution of demographic and clinical variables were assessed using the χ2 test or Fisher's exact test. Median follow-up was calculated for patients alive at last follow-up.
Survival analysis for OS, EFS, and DFS was performed using the Kaplan-Meier method, and comparisons between groups were performed using the log-rank test. Univariate and multivariate analyses were performed using the Cox regression proportional hazards model. The following binary variables were included in univariate analysis: sex, age ≥ 45 years, stage III or IV, WBC count ≥ 15 × 109/L, lymphocyte count less than 0.6 × 109/L, hemoglobin less than 10.5 g/dL, albumin less than 40 g/L, histologic subtype, presence of B symptoms, BM involvement, and HIV status. Adjusted models were constructed using prespecified prognostic variables. Univariate and multivariate analyses for all outcomes were repeated including HIV status and IPS as a score (0 to 2 v ≥ 3), rather than the individual variables that compose the IPS. A logistic regression model was used to estimate the effect of HIV status on response rate after adjusting for covariates. Statistical significance was set as P < .05. All statistical analyses were performed using STATA version 11 (STATA, College Station, TX) and SPSS version 16.0 (SPSS, Chicago, IL).
RESULTS
Response to Therapy
Forty-six patients (including eight HIV-positive patients) received IFRT because of early-stage disease (n = 15; HIV positive, n = 6), bulky disease at diagnosis (n = 21; HIV positive, n = 1), persistence of residual masses after completing chemotherapy (n = 7; HIV positive, n = 1), or a perceived high-risk disease by the attending physician (n = 3, all HIV negative). The response after completing treatment (including IFRT in patients who received it) was as follows: CR/Cru in 172 patients (77%), partial response in 33 patients (15%), and stable disease/progression in 18 patients (8%). One patient with HIV infection died of neutropenic sepsis after the last cycle and was not assessable for response (Table 2). The only variable associated in univariate analysis with achieving a CR/CRu was stage III or IV (P = .02). HIV status and IPS (0 to 2 v ≥ 3) did not impact on the response rate. Multivariate analysis including HIV status and the variables composing the IPS did not identify any significant factors associated with response.
Table 2.
Response to Treatment After ABVD Chemotherapy With or Without IFRT
| Response | HIV-Positive Patients |
HIV-Negative Patients |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| CR/CRu | 69 | 74 | 103 | 79 | .34 |
| PR | 16 | 17 | 17 | 13 | |
| SD/progression | 7 | 8 | 11 | 8 | |
| Toxic death | 1 | — | — | ||
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; CR, complete response; CRu, complete response uncertain; IFRT, involved-field radiotherapy; PR, partial response; SD, stable disease.
Relapse and DFS
After a median follow-up of 60 months (range, 8 to 174 months), 16 HIV-negative patients (16%) and seven HIV-positive patients (10%) in CR/CRu after treatment experienced relapse. The median time to relapse was 7 months (range, 1 to 108 months) for HIV-negative patients and 5 months (range, 1 to 37 months) for HIV-positive patients. Five-year DFS was 85% (95% CI, 76% to 91%) and 87% (95% CI, 74% to 94%) for HIV-negative and HIV-positive patients, respectively (P = .5). Stage III or IV disease was associated with a trend toward a shorter DFS on univariate analysis (P = .07). No variables retained predictive value on multivariate analysis. After adjusting for IPS, HIV remained nonsignificant for DFS.
OS and EFS
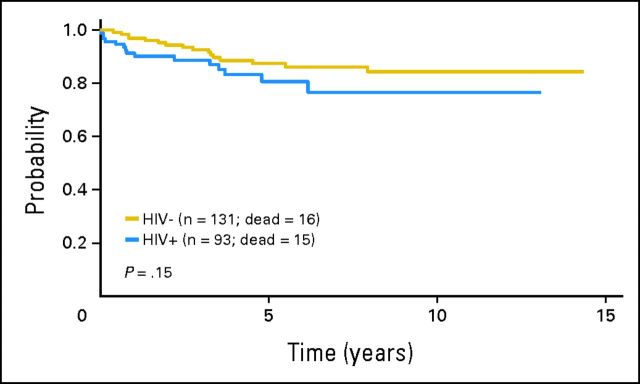
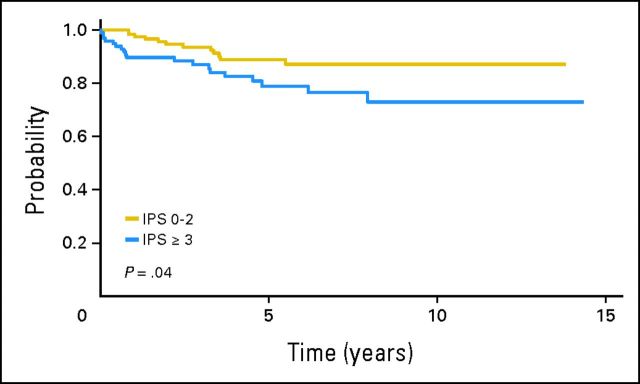
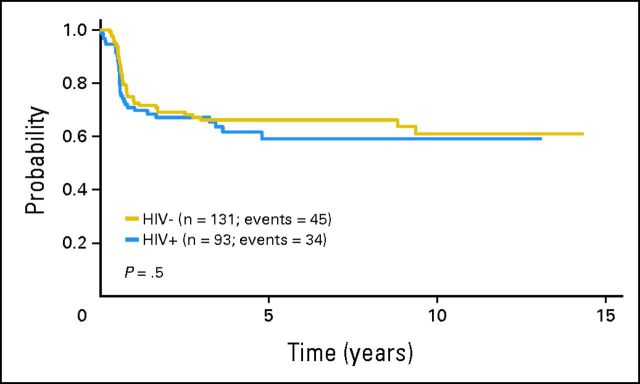
Sixteen HIV-negative patients and 15 HIV-positive patients have died at a median time of 32 months (range, 5 to 97 months) and 9 months (range, 1 to 75 months), respectively. The cause of death for HIV-negative patients was HL in 15 patients and cardiac cause in one patient. Among HIV-positive patients, 10 patients died of HL, one patient died of neutropenic sepsis after the last cycle of chemotherapy, one patient died of an opportunistic infection 1 year after the diagnosis of HL, and three patients died of other unrelated causes (pancreatic adenocarcinoma, stroke, and heart attack; n = 1 each). Five-year OS for HIV-negative patients was 88% (95% CI, 80% to 93%), with no significant differences compared with HIV-positive patients (81%; 95% CI, 69% to 89%; P = .15; Fig 1). The variables associated with a shorter OS on univariate analysis were the following: stage III or IV disease, low hemoglobin level, presence of B symptoms, presence of BM involvement, and IPS ≥ 3 (Fig 2). On multivariate analysis including HIV status, the only variable associated with a shorter OS was high-risk IPS (hazard ratio, 1.973; 95% CI, 0.9 to 4.326; P = .09). Five-year EFS was 66% (95% CI, 57% to 74%) and 59% (95% CI, 47% to 70%) for HIV-negative and HIV-positive patients, respectively (P = .5; Fig 3). Advanced stage, low hemoglobin, presence of B symptoms, and presence of BM involvement were associated with a shorter EFS in the univariate analysis. Only stage III or IV disease retained predictive value for EFS on multivariate analysis including HIV status. IPS did not predict EFS on multivariate analysis.
Fig 1.
Overall survival according to HIV status.
Fig 2.
Overall survival according to the International Prognostic Score (IPS; 0 to 2 v 3 to 7).
Fig 3.
Event-free survival according to HIV status.
Outcome According to HIV Status in Patients With Advanced-Stage HL
There were no differences in DFS, EFS, or OS according to HIV status in patients with advanced-stage HL. Five-year DFS for HIV-negative patients was 84% (95% CI, 74% to 91%) compared with 88% (95% CI, 75% to 94%) in HIV-positive patients (P = .6). No variables retained predictive value on multivariate analysis. After adjusting for IPS, HIV serostatus remained nonsignificant for DFS. The OS rates at 5 years were 85% (95% CI, 77% to 91%) and 83% (95% CI, 71% to 90%) for HIV-negative and HIV-positive patients, respectively (P = .6). On multivariate analysis including HIV status, the only variable associated with a shorter OS was high-risk IPS (hazard ratio, 2.044; 95% CI, 0.9 to 4.564; P = .08). Five-year EFS rates for HIV-negative and HIV-positive patients were 64% (95% CI, 54% to 73%) and 64% (95% CI, 53% to 74%), respectively. After adjusting for IPS, HIV remained nonsignificant for EFS.
DISCUSSION
This large retrospective study with a long follow-up demonstrates for the first time, to our knowledge, that in the HAART era, the outcome of HL in patients with HIV infection treated with the standard ABVD regimen is comparable to that of HL in the general population. The incidence of HL, a non–AIDS-defining malignancy with an unclear relationship with the CD4 count, is, nonetheless, significantly increased in the HIV population.1,2 In contrast with other types of lymphoma where incidence increases in patients with lower CD4 counts, some studies have shown that the incidence of HL is lower in patients with severe immunosuppression than in those with moderate immunosuppression.4,18 There is also evidence in some series, but not confirmed in others,6–8 that the incidence of HL is increasing in the HAART era.4,5 The basis for this is unclear, but if confirmed, we should expect to see more HIV-positive patients diagnosed with HL in the years to come.
The clinical characteristics of HL in patients with HIV infection have been compared with those of NHL in patients with HIV infection.19 The proportion of patients with aggressive clinical features at diagnosis of HL has been shown to be higher in the HIV population than in HIV-negative patients, and this observation has been confirmed in the current series. Eighty percent of HIV-positive patients had stage III or IV disease at diagnosis, compared with 35% of HIV-negative patients, and in 68% of HIV-positive patients, the IPS was ≥ 3. The IPS (developed for patients with advanced-stage HL) has demonstrated its predictive value in HIV-negative patients20 and in HIV-positive patients treated with the Stanford V regimen,21 but not in patients with HIV infection treated with ABVD.22 In the current study, IPS ≥ 3 was associated with a significantly shorter OS, confirming that a high-risk IPS confers a poor prognosis in this population.
Despite the poor-risk features of HL in the HIV population, this study demonstrates for the first time that their outcome is comparable to that in HIV-negative patients when treated with the same regimen (ABVD). Five-year OS rates were 88% and 81% in HIV-negative and HIV-positive patients, respectively, and 5-year EFS rates were 66% and 59%, respectively, with no significant differences according to HIV status. The largest series in 62 HIV-positive patients treated with ABVD reported a 5-year OS of 76%, similar to the results in the current study.23 Another study reporting on the Stanford V regimen in 59 patients with HIV and HL showed 3-year OS and DFS rates of 51% and 68%, respectively.21 The more intensive bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) regimen has also been used in a small series of 12 HIV-positive patients, but two patients treated in the pre-HAART era died of opportunistic infections within the treatment period,24 and a more recent study, presented only in abstract form, has reported considerable toxicity in HIV-positive patients receiving BEACOPP.25 However, none of these studies have compared the outcome of patients with HL treated with the same chemotherapy regimen according to HIV status. In contrast, several studies have shown that the outcome of patients with NHL and HIV infection in the HAART era approaches that of HIV-negative patients when treated with the same protocols. The outcome of patients with HIV and NHL was dismal before the introduction of HAART, and patients were frequently treated with regimens that would have been considered palliative in the general population.26 The introduction of HAART and the increased experience in using standard chemotherapy regimens and better supportive therapy27 have resulted in the clear demonstration that even intensive chemotherapy protocols are feasible in patients with HIV28 and that the outcome of HIV-positive patients with Burkitt's lymphoma11 and diffuse large B-cell lymphoma10 is similar to that of HIV-negative patients when they received the same chemotherapy regimens. This study demonstrates for the first time, to our knowledge, that the same applies to HL.
The objective of this study was to analyze the outcome of HIV-positive patients with cHL deemed by their treating physicians to be fit enough to receive ABVD and to compare it with the outcome of this regimen in the general population, rather than to describe the prognosis of HIV-positive patients with cHL, because this has been previously reported. Hence, as in any study on the outcome of patients after a specific treatment, there is an inherent selective bias in this series. However, such bias affects both groups, as demonstrated by the fact that in the study period, 27% of HIV-negative patients were treated with regimens other than the standard ABVD and this included 8% of patients who were considered too frail to receive ABVD. Nevertheless, the HIV-positive patients had significantly more poor-risk features than the HIV-negative patients, supporting the fact that the selection bias did not result in a better risk group for HIV patients, which would invalidate the main conclusion of this study.
One of the caveats of the present study is the lack of detailed prospectively recorded data on toxicity, dose reductions, and treatment delays, on account of its retrospective nature. Chemotherapy delivery in HIV-seropositive patients has been associated with greater toxicity and thus delays in the delivery of treatment.29 However, even the most intensive regimens are feasible if patients receive appropriate supportive therapy.
Despite the increasingly widespread use of concomitant HAART during chemotherapy, this approach remains controversial, with some centers stopping HAART during chemotherapy for NHL to avoid increased toxicity.30 Nevertheless, in patients with HIV receiving chemotherapy for HL, both the use of HAART and the response to HAART have been associated with a better outcome.23,31 In the current study, 92 of 93 HIV-positive patients received HAART concomitantly with ABVD, and only one HIV-positive patient died of treatment-related toxicity. The lack of differences in OS and EFS between HIV-positive and HIV-negative patients suggests that concomitant treatment with HAART neither increases the fatal toxicity of patients treated with ABVD nor jeopardizes their outcome.
In summary, this study consolidates the increasingly prevalent notion that, in the current HAART era, patients with HIV and lymphoma should be treated with the same protocols used in HIV-negative patients. As a corollary, HIV status should be removed from the exclusion criteria for entry onto clinical trials.
Footnotes
Supported by grants from the Olivia Walduck Family and the Mark Ridgwell Family Trust (S.M.).
Presented in part at the 53rd Annual Meeting of the American Society of Hematology, December 10-12, 2011, San Diego, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Silvia Montoto, Roche, Celgene; Chloe Orkin, Janssen, Gilead, Bristol-Myers Squibb, Boehringer Ingelheim, MSD, GlaxoSmithKline, Viiv; John G. Gribben, Roche, Celgene, GlaxoSmithKline, Mundipharma, Gilead, Pharmacyclics Research Funding: Silvia Montoto, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Silvia Montoto, Kate Cwynarski, Robert Marcus, Mark Bower
Provision of study materials or patients: Silvia Montoto, John G. Gribben, Mark Bower
Collection and assembly of data: Silvia Montoto, Kate Shaw, Jessica Okosun, Shreyans Gandhi, Paul Fields, Andrew Wilson, Kate Cwynarski, Robert Marcus, Johannes de Vos, Anna Marie Young, Melinda Tenant-Flowers, Chloe Orkin, Margaret Johnson, Daniella Chilton, Mark Bower
Data analysis and interpretation: Silvia Montoto, Milensu Shanyinde, Kate Cwynarski, Robert Marcus, John G. Gribben, Mark Bower
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Cole SR, Kirk GD, et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggar RJ, Chaturvedi AK, Goedert JJ, et al. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 4.Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 6.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 7.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989-2002. Clin Infect Dis. 2004;39:1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 8.Seaberg EC, Wiley D, Martinez-Maza O, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116:5507–5516. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- 10.Navarro JT, Lloveras N, Ribera JM, et al. The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologica. 2005;90:704–706. [PubMed] [Google Scholar]
- 11.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: Comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113:117–125. doi: 10.1002/cncr.23522. [DOI] [PubMed] [Google Scholar]
- 12.Gazzard BG, Anderson J, Babiker A, et al. British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. doi: 10.1111/j.1468-1293.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 13.Bower M, McCall-Peat N, Ryan N, et al. Protease inhibitors potentiate chemotherapy-induced neutropenia. Blood. 2004;104:2943–2946. doi: 10.1182/blood-2004-05-1747. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 15.Santoro A, Bonadonna G, Valagussa P, et al. Long-term results of combined chemotherapy- radiotherapy approach in Hodgkin's disease: Superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27–37. doi: 10.1200/JCO.1987.5.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 18.Lanoy E, Rosenberg PS, Fily F, et al. HIV-associated Hodgkin lymphoma during the first months on combination antiretroviral therapy. Blood. 2011;118:44–49. doi: 10.1182/blood-2011-02-339275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Re A, Casari S, Cattaneo C, et al. Hodgkin disease developing in patients infected by human immunodeficiency virus results in clinical features and a prognosis similar to those in patients with human immunodeficiency virus-related non-Hodgkin lymphoma. Cancer. 2001;92:2739–2745. doi: 10.1002/1097-0142(20011201)92:11<2739::aid-cncr10121>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease: International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 21.Spina M, Gabarre J, Rossi G, et al. Stanford V regimen and concomitant HAART in 59 patients with Hodgkin disease and HIV infection. Blood. 2002;100:1984–1988. doi: 10.1182/blood-2002-03-0989. [DOI] [PubMed] [Google Scholar]
- 22.Xicoy B, Ribera JM, Miralles P, et al. Limited prognostic value of the International Prognostic Score in advanced stage human immunodeficiency virus infection-related Hodgkin lymphoma treated with the doxorubicin, bleomycin, vinblastine, and dacarbazine regimen. Leuk Lymphoma. 2009;50:1718–1720. doi: 10.1080/10428190903174359. [DOI] [PubMed] [Google Scholar]
- 23.Xicoy B, Ribera JM, Miralles P, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus-related Hodgkin's lymphoma. Haematologica. 2007;92:191–198. doi: 10.3324/haematol.10479. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann P, Rehwald U, Salzberger B, et al. BEACOPP therapeutic regimen for patients with Hodgkin's disease and HIV infection. Ann Oncol. 2003;14:1562–1569. doi: 10.1093/annonc/mdg408. [DOI] [PubMed] [Google Scholar]
- 25.Hentrich M, Berger M, Hoffmann C, et al. HIV-related Hodgkin's lymphoma (HIV-HL): Results of prospective multicenter trial. Haematologica. 2011;96:s2. (abstr 519) [Google Scholar]
- 26.Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin lymphoma: Final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–3840. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- 27.Bower M, Collins S, Cottrill C, et al. British HIV Association guidelines for HIV-associated malignancies 2008. HIV Med. 2008;9:336–388. doi: 10.1111/j.1468-1293.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 28.Montoto S, Wilson J, Shaw K, et al. Excellent immunological recovery following CODOX-M/IVAC, an effective intensive chemotherapy for HIV-associated Burkitt's lymphoma. AIDS. 2010;24:851–856. doi: 10.1097/QAD.0b013e3283301578. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein PG, Braik T, Jain S, et al. Ritonavir based highly active retroviral therapy (HAART) correlates with early neurotoxicity when combined with ABVD treated HIV associated Hodgkin lymphoma but not Non-Hodgkin lymphoma: A retrospective study. Blood. 2010;116:2807. (abstr) [Google Scholar]
- 30.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: Impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 31.Hentrich M, Maretta L, Chow KU, et al. Highly active antiretroviral therapy (HAART) improves survival in HIV-associated Hodgkin's disease: Results of a multicenter study. Ann Oncol. 2006;17:914–919. doi: 10.1093/annonc/mdl063. [DOI] [PubMed] [Google Scholar]