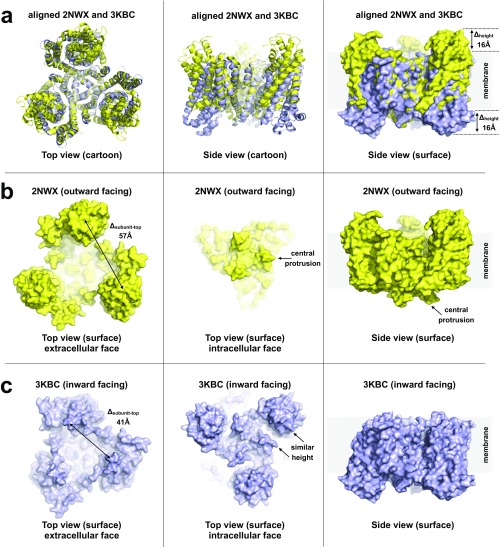
Fig. S3.
Analysis of key structural features of and conformational changes between the high-resolution structures of the outward- and inward-facing states. (A) Superposition of the crystal structures of the symmetric outward-facing (PDB ID code 2NWX, yellow) and inward-facing (PDB ID code 3KBC, blue) states of GltPh. Part of the static central scaffold domain (residues 152–199) was used for alignment. These regions (576 atoms) aligned in four cycles to a rms of 0.5 Å using PyMol. (Left and Center) Cartoon representation of the aligned structures viewed from the extracellular face and in the membrane plane, respectively. (Right) Surface representation of the superposed structures; the elevator domains are significantly shifted (∼16 Å) in the direction normal to the membrane plane as measured on the positions of the most outward- and inward-facing residues N378 and S295. (B) Outward-facing structure (PDB ID code 2NWX) viewed from the extracellular space (Left). The three elevator domains protrude significantly from the membrane, forming a triangular topography with top-to-top distances of ∼57 Å (measured on N378). Viewed from the cytoplasm (Center), the trimer scaffold domain protrudes most (beginnings of the long helices 5, residue K175). Viewed from the side (Right), the central scaffold domain protrudes from the membrane on the intracellular face. (C) Inward-facing structure (PDB ID code 3KBC) viewed from the extracellular space (Left). The highest protrusions (residues Q120) are separated by ∼41 Å. Elevator domains do not protrude significantly from the membrane in this conformation; see side view (Right). Viewed from the intracellular face (Center), the elevator domains protrude (residue S295) just slightly more than the central scaffold domain (residue K175).