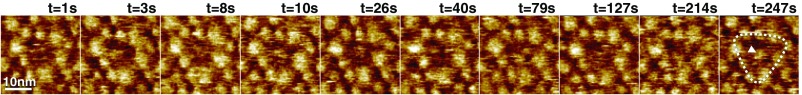
Fig. S4.
GltPh trimer viewed from the cytoplasmic face. Ten representative HS-AFM frames of the inward-facing side of GltPh revealing domain motion; full color scale is 3 nm. GltPh transporter reconstitution into lipid vesicles and subsequent adsorption to the HS-AFM sample support favored exposure of the extracellular face of GltPh for HS-AFM analysis (Fig. 1A). Occasionally, trimers exposing their cytoplasmic side to the HS-AFM tip were imaged. In agreement with the predictions from the X-ray structures (Fig. S3), this face exposed less-pronounced surface features and appeared globally as darker (less protruding) triangles (outlined by dashed line in frame t = 247 s). The central protrusion of the trimerization domains is most conspicuous when the elevator domains are all in the outward-facing state (e.g., frame t = 10 s and t = 26 s, indicated by arrowhead in frame t = 247 s). HS-AFM could detect transport domain motions to the inward-facing state from the cytoplasmic side (e.g., frame t = 79 s, in which clearly two individual protrusions are visible), but it was not possible to assign elevator states with certainty (Movie S4), and only molecules exposing the extracellular face to the HS-AFM tip were integrated into the analysis.