Abstract
Nasopharyngeal carcinoma (NPC) is a head and neck cancer that represents a major health burden in Southern China and Southeast Asia. Although the close association of NPC with Epstein–Barr virus (EBV) infection has been demonstrated, its exact role in the pathogenesis of this malignancy is still unclear. The expression of EBV-encoded microRNAs, especially BART miRNAs, which are encoded from the BamHI-A region of the viral genome, is detected at a high level in NPC. miRNAs are small noncoding mRNAs that can positively regulate the virus to ensure accurate expression of viral genomes and to modify the gene expression of host cells by negative regulation. Accumulating evidence suggests that ebv-mir-BARTs play a critical role in host cell survival, immune escape, cell proliferation, cell apoptosis, and cancer metabolism, promoting the generation of NPC. This review will summarize our current understanding of the nature and function of ebv-mir-BARTs in NPC.
Keywords: BART, Epstein–Barr virus, microRNA, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is a prevalent malignancy with a high incidence in southern China. The differences in the geographic and ethnic distributions reflect the multifactorial etiology of NPC, including Epstein–Barr virus (EBV) infection, genetic susceptibility, and environmental factors. According to the new classification of the WHO, NPC is classified into two histological subtypes: keratinizing squamous cell carcinoma and nonkeratinizing carcinoma (either differentiated or undifferentiated) (Tsao et al., 2015); the latter accounts for more than 97% of this cancer and is consistently associated with EBV infection (Chou et al., 2008), suggesting that EBV is a crucial factor in the pathogenesis of NPC.
EBV is an oncogenic virus, latently infecting more than 90% of the global population (Cai et al., 2015b), and is closely linked to several human malignancies including Burkitt’s lymphoma, Hodgkin’s disease, post-transplant lymphoma, extranodal nasal natural killer/T-cell lymphoma (NKTCL), EBV-associated gastric cancers (EBVaGCs), and NPC (Hsu et al., 2014; Tsao et al., 2015). Both lytic and latent EBV genes may be involved in the tumorigenesis of human carcinoma, causing at least 200 000 new cancers per year (Kuzembayeva et al., 2014; Cai et al., 2015c). EBV shuttles continuously between B cells and epithelial cells during its infection cycle, maintained by a check-and-balance of the immune system in the body (Tsang and Tsao, 2015), which may be critical to establishing a persistent infection in humans. Once this intricate balance is broken, an uncontrolled proliferation of EBV-infected cells occurs that can induce malignancies. It is now well known that EBV facilitates the pathogenesis of NPC (Gu et al., 2012); several EBV genome products such as viral proteins, RNAs, and miRNAs may participate in the development of NPC (Raab-Traub, 2002). EBNA1, an EBV-encoded protein, promotes DNA damage in NPC cells by reducing P53 levels and inducing reactive oxygen species (ROS) (Raab-Traub, 2002; Gu et al., 2012; Tulalamba and Janvilisri, 2012). ROS may oxidatively modify miRNA and result in alteration of cellular events (Wang et al., 2015). LMP1 and LMP2B have a close association with cell proliferation, apoptosis, invasion, and motility by regulating several signaling pathways to influence the formation of vascular channels, alter the microenvironment of cancer and so on. However, the pathogenesis and progression of NPC are not only regulated through EBV proteins but also by EBV-encoded miRNAs. Currently, ebv-miRNA, a short single-stranded RNA molecule that post-transcriptionally regulates gene expression, has generated major concern.
To date, a total of 48 mature miRNAs have been identified. These miRNAs are transcribed as two regions: BamHI fragment A rightward transcript (BART) and BamHI fragment H rightward reading frame 1 (BHRF1) in the EBV genome (Raab-Traub, 2002; Gu et al., 2012; Tsang and Tsao, 2015). Of these, BART miRNAs are highly expressed in epithelial tumors of NPC and EBVaGC, regulating the expression of various genes at the post-transcriptional level (Lo et al., 2012; Marquitz and Raab-Traub, 2012). At least 105 host genes are regulated by ebv-miRNA in NPC development, influencing five significant signaling pathways including the Wnt signaling pathway (Zeng et al., 2014), which is the major pathway, and its regulation by EBV is not dependent on the expression of LMP1 (Webb et al., 2008). Evidence of the underlying carcinogenesis of ebv-mir-BARTs is accumulating rapidly. In this review, we will present a summary of the nature and function of ebv-mir-BARTs in NPC (Table 1).
Table 1.
Function of BART miRNAs in nasopharyngeal carcinoma
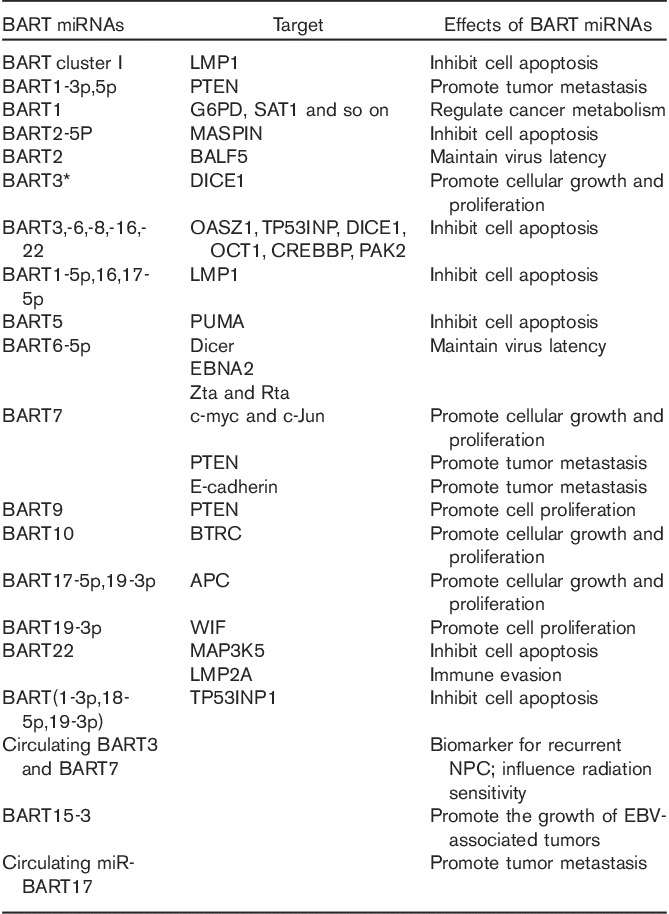
BART miRNAs contribute toward virus latency in NPC
During the infection cycle of EBV, EBV establishes persistent infection in humans by maintaining a check-and-balance of the immune system. The ebv-miRNA, a key player in establishing and maintaining latency, functions as a regulator of the viral life cycle.
ebv-miR-BART2
Important results have been reported on the impact of ebv-miR-BART2 on the transition from latent to lytic infection. It serves as an inhibitor of viral DNA replication through complete complementary with the 3′UTR of BALF5 (Barth et al., 2008), degrading mRNA and inhibiting transition from latent to lytic viral replication. BALF5 can be defined as a DNA polymerase that can replicate viral genomic DNA in the late phase of lytic infection, producing long concatemeric DNA. However, mir-BART2 is only expressed at very low levels during latent infection; we suggest that other factors regulate BALF5 mRNA to ensure viral replication.
ebv-mir-BART6-5p
In addition to BART2, regulation of BART6 may be essential for EBV latent infection. First, ebv-mir-BART6-5p (Iizasa et al., 2010) silences Dicer, which is also regulated by miR103/107 and let-7, affecting the state of virus latency. Furthermore, BART6, an EBV-encoded miRNA, suppresses EBNA2, which is required for the transition of latency types. EBNA2 may be involved in the transactivation of LMP1 and, by inhibiting EBNA2, the virus can remain in a specific state of latency. Finally, BART6 inhibits essential viral proteins such as Zta and Rta, which are important for viral lytic replication. Another independent study reported that either adenosine-to-inosine (A-to-I) editing or mutations of BART6 influence the maintenance of latent EBV infection.
BART miRNAs are closely associated with cell growth and proliferation in NPC
BART miRNAs play physiologic and pathophysiologic roles in cell proliferation, providing novel insight into the relationship between malignancies and EBV. Accumulating evidence indicates that deregulation of specific miRNAs is associated with cancer initiation and progression in NPC and that they can function as oncogenes to promote cell proliferation.
ebv-mir-BART3*
A couple of viral and cellular targets of EBV-miRNAs in NPC cells have been validated completely. Of these, BART3* was found to promote cellular growth and transformation in NPC by matching imperfectly with DICE1 (Lei et al., 2013a). DICE1, a tumor suppressor, has growth-suppressing activity and interferes with anchorage-independent growth of IGF-IR-transformed tumor cells. Although the inhibitory effect of miR-BART3* might be mild, the level of DICE1 expression is high in NPC and it may be crucial in promoting cell proliferation in the initiation of NPC tumorigenesis. Similar to BART6, A-to-I editing (Lei et al., 2013b) may interfere with the target of miR-BART3 and induce changes in its expression and function in EBV-infected epithelial cells such as NPC and EBVaGC.
ebv-mir-BART9
ebv-mir-BART9 exerts a tumorigenesis effect by targeting E-cadherin to promote cell invasion (Hsu et al., 2014) and is involved in cell proliferation by targeting a key tumor suppressor, PTEN. PTEN is a protein tyrosine phosphatase that participates in the process of PI3K activation, an abnormal proliferation signaling pathway that contributes toward tumorigenesis of NPC. Although downregulation of PTEN is found in about half of NPC tumors, mutation of the PTEN gene is extremely rare in HNSCC and its promoter hypermethylation has not been investigated in NPC (Chou et al., 2008; Tulalamba and Janvilisri, 2012). Therefore, other mechanisms are likely responsible for the low PTEN levels, such as ebv-mir-BARTs. ebv-mir-BART9 has been found to target PTEN to promote cell proliferation, although more evidence is needed to confirm its function in NPC.
ebv-mir-BART10
As is well known, regulation of cell-cycle checkpoints may result in tumorigenesis. Results obtained by Zeng et al. (2014) indicated that ebv-mir-BART10 can efficiently suppress the expression of BTRC and subsequently decrease degradation of its substrates such as β-catenin and Snail, regulating the development of NPC. β-Catenin is the key mediator of canonical signaling in the Wnt pathway. Aberrant activation of the Wnt pathway results in the cytoplasmic β-catenin translocating into the nucleus, thereby interacting with various transcription factors to promote specific gene expression, causing cellular proliferation. miRNA has emerged as a new factor that indirectly regulates the Wnt signaling pathway by targeting β-catenin. WIF and APC, two key Wnt inhibitory genes also targeted by ebv-mir-BART19-3p and ebv-mir-BART17-5p,19-3p, respectively (Jiang et al., 2011; Wong et al., 2012; Xie et al., 2013), regulate the Wnt pathway synergistically.
ebv-mir-BART7
A number of studies have evaluated the role of ebv-mir-BART7, discovering that ebv-mir-BART7 also enhances cell growth, colony formation, and cell-cycle progression by activating PI3K/Akt/c-myc and c-Jun, eventually promoting cell proliferation and tumorigenesis (Chan et al., 2012; Cai et al., 2015a). c-Myc and c-Jun transcription factors are critical promoters of cellular proliferation, especially c-Myc; increased expression was found in 90% of NPC, sequestering inhibitory p27 and allowing for cell proliferation and progression through the G1 phase. Moreover, evidence from Chan et al. (2012) showed that BART7 alters multiple cancer-related pathways in undifferentiated NPC cells, suggesting that BART7 plays a crucial role in the development of NPC. Use of a gold–PEI nanocarrier to deliver anti-miR-BART7-3p can inhibit the function of BART7 (Cai et al., 2015c). Although nanoparticles may have toxicity and instability, they represent a new therapeutic method based on miRNA.
miR-BART8
As mentioned above, ebv-mir-BARTs are closely associated with cell growth and proliferation in NPC. However, there are very few investigations on the functions of BART miRNAs. Huang and Lin (2014) reported that EBV-encoded miR-BART8 inhibits the IFN-γ-STAT1 pathway with secondary suppression of TP53, which may promote tumor growth in nasal NK-cell lymphoma. However, the role of miR-BART8 in NPC is not clear; further study is warranted to understand the role of mir-BARTs in the NPC.
BART miRNAs may play a crucial role in the cell apoptosis in NPC
Inhibition of apoptosis is critical for development of malignancies, including NPC. Survivin overexpression and high telomerase activity contribute toward NPC cell immortalization and overexpression of ebv-mir-BARTs, which play an essential role in preventing apoptosis in NPC. Some well-defined examples are BART1-5p,16,17-5, ebv-mir-BART5, and BART cluster I.
BART1-5p,16,17-5p
A number of studies have evaluated the role of ebv-mir-BART1-5p,16,17-5p in apoptosis resistance by targeting LMP1 in epithelial carcinoma including NPC, which plays a role in tumor initiation (Cai et al., 2015b). LMP1 is believed to play a crucial role in the development of NPC (Dawson et al., 2012), but LMP1 protein expression in NPC is variable. In precancerous lesions, a high level of LMP1 expression triggers its oncogenic functions, whereas a relatively low level occurs in advanced cancer. LMP1 is downregulated by ebv-mir-BART1-5p,16,17-5p to prevent its cytotoxic effects and makes NPC cells resistant to apoptosis.
BART cluster I
LMP1 is a target of ebv-mir-BART1-5p,16,17-5p as well as BART cluster I. BART cluster I negatively regulates NF-κB activity in NPC by regulating LMP1 at the level of post-transcription (Lo et al., 2007). NF-κB dysregulation is one of the most important components of NPC tumorigenesis, indicated by the fact that it modulates cell proliferation and inflammation in carcinogenesis of numerous neoplasms. Activation of NF-κB, promoting the release of cytokines from inflammatory cells, may contribute toward the malignant transformation of premalignant epithelial cells; upregulation of inflammatory cytokines would continue to cause activation of NF-κB, generating a positive loop (Chou et al., 2008; Tulalamba and Janvilisri, 2012). Moreover, activation of NF-κB may cause accumulation of p53, which may be advantageous to the development of NPC by preventing JNK-induced apoptosis. Yet, NF-κB also activates a number of proliferative signals, including Bcl-2. Another independent study showed that BART cluster I also targets the Bcl-2 interacting mediator of cell death (Bim), exerting an effect on the inhibition of apoptosis in response to the chemical compound etoposide in the AGS gastric carcinoma cell line (Marquitza et al., 2011). Similarly, it inhibits cisplatin-induced cytotoxicity by directly modulating NF-κB and indirectly regulating telomerase activity and cell immortalization in NPC; finally, it may result in LMP1-induced immortalization, suggesting that tumor cell apoptosis is significantly governed by ebv-mir-BARTs.
ebv-mir-BART5
p53 upregulated modulator of apoptosis (PUMA) is a proapoptotic member of the BH3-only subgroup of the Bcl-2 family. It is a key mediator of p53-dependent and p53-independent apoptosis, having the strongest proapoptotic effects found in recent years (Hikise and Kilianska, 2012). PUMA protein expression, as well as PUMA mRNA, can be detected in cells with virus infection. Previous findings reported that PUMA is a core factor that considerably improved the sensitivity to chemotherapy drugs and radiation. Radioresistance and growth inhibition may be ascribed to the knockdown of PUMA in lung cancer, breast cancer, and other malignancies. For NPC, studies found that PUMA was targeted by ebv-mir-BART5 (Choy et al., 2008). BART5 downregulates the expression of PUMA, protecting host cells from apoptosis.
ebv-mir-BART20
The Bcl-2-associated death promoter has been reduced at the level of both mRNA and protein by ebv-mir-BART20-5p in EBaGC cells (Kim et al., 2015), enhancing cell growth, suppressing cell apoptosis, and inducing chemoresistance to 5-FU and docetaxel. In addition, BART20-5p targets BZLF1 and BRLF1 (Jung et al., 2014), which may trigger the lytic cycle and tightly regulate EBV latency in the EBV-infected gastric carcinoma cell line. Furthermore, mir-BART20-5p may indirectly inhibit p53 in invasive nasal NK/T-cell lymphoma (Lin et al., 2013). Both EBaGC and NPC belong to EBV latency type II; in particular, the level of BART20 in NPC is higher than that of EBaGC. Therefore, we suggest that BART20 may play a more important role in tumorigenesis initiation of NPC than that of EBaGC, influencing the chemosensitivity of cancer cells, the immune response of host cells, and the latency of EBV.
BART miRNAs may induce tumor metastasis and recurrence in NPC
Although IMRT has improved the survival rate of NPC significantly, it still has a propensity for distant metastasis, which is one of the main causes of treatment failure. In addition to EBV latency proteins (LMP1, LMP2A), EBV-encoded miRNAs also play a critical role in the tumor metastasis in NPC.
ebv-mir-BART1, BART7
Considerable evidence has shown that downregulation of PTEN is responsible for upregulation of the PI3K/Akt pathway in NPC. In addition, loss of PTEN is associated with metastatic disease, with clinical stage III–IV tumors having lower PTEN than clinical stage I–II tumors (Chou et al., 2008; Tulalamba and Janvilisri, 2012). In-vitro and in-vivo studies have reported that BART1 (BART1-3p,BART1-5p) can directly target PTEN, activate PTEN-dependent pathways, lead to EMT, and finally contribute toward metastasis (Cai et al., 2015b). EMT, a fundamental biological process that enhances the capacity for cell migration and invasion, is considered the initial step of tumor metastasis. Similarly, ebv-mir-BART7-3p also targets suppressor PTEN (Chan et al., 2012; Cai et al., 2015a), modulating PI3K/AKT/GSK-3β signaling and inducing a transition from the epithelial to the mesenchymal phenotype by promoting EMT, eventually leading to cancer metastasis.
ebv-mir-BART9
E-cadherin, a transmembrane glycoprotein that functions as a cell adhesion molecule, is essential for the morphogenesis and homeostasis of epithelial tissues. Low E-cadherin expression is associated significantly with metastasis in head and neck cancer patients, playing a key role in EMT by inducing transcription factors such as Twist. As for NPC, lower E-cadherin mRNA and protein levels are found in metastatic NPC tumors, suggesting that decreased E-cadherin levels correlate with metastatic disease (Wong et al., 2014). To date, three main factors account for the low expression of E-cadherin: loss of β-catenin in the cytoplasm, promoter hypermethylation, and microRNA. It has been reported that ebv-mir-BART9 directly downregulates E-cadherin at the post-transcriptional level (Lo et al., 2012; Hsu et al., 2014), whereas LMP1 regulates E-cadherin at the transcriptional level, suggesting that these two viral products may repress E-cadherin synergistically in NPC. It was discovered that BART9 may regulate the level of LMP1 and affect the growth rate in NKTCL (Ramakrishnan et al., 2011). We can surmise that BART9 targets LMP1 in NPC to generate motile cells, inducing cell invasion and migration. Unfortunately, it has been found that the effect of BART9 is independent of LMP1, LMP2A, and EBNA1.
ebv-mir-BART7
As is well known, local recurrence is another factor that contributes toward treatment failure in NPC. Surgery may play a more important role in local tumor control and survival compared with reradiation for recurrent patients. mir-BART7 is present in high levels in plasma and tumor in patients with NPC, especially in the advanced stage. It may predict and identify patients with histologically clear margins (>5 mm) who may have an increased risk of local recurrence after resection of recurrent NPC (Chan et al., 2015). In addition, mir-BART7 may influence radiation sensitivity, contributing toward altered treatment outcomes.
Regulation of cancer metabolism by BART miRNAs
Metabolic pathway alterations, including aerobic glycolysis and the Warburg effect in cancer cells, are also observed in NPC. BART miRNAs can participate in cancer cell metabolism by regulating the expression of metabolism-associated genes whose protein products regulate metabolism either directly or indirectly. Ye et al. (2013) reported, using both assays in cells and NPC tissues, that ebv-mir-BART1 could significantly influence the expression of G6PD, SAT1, and other key genes associated with metabolism. Notably, PAST1 and PHGDH appeared to be upregulated mostly in NPC; they may also be critical for the proliferation and growth of cancer cells, respectively.
Immune evasion function of BART miRNAs in NPC
It is well known that the existence of EBV may have less opportunity to induce host immune response, promoting tumor cell survival. ebv-mir-BART21 and ebv-mir-BART22 are highly expressed in NPC cell lines and primary tissues. Importantly, BART22 can downregulate LMP2A at the level of translation and eventually escape the host immune response (Lung et al., 2009), promoting the survival of cancer cells of NPC. Newer data show that LMP2, especially LMP2A, a potent immunogenic viral antigen, has diverse functions including mediation of tumor cell survival and inducing NPC cells to become migratory and invasive (Chou et al., 2008; Dawson et al., 2012). BART2-5P and BART3 can target MICB and IPO7 (Nachmani et al., 2009), respectively, escaping immune recognition and killing in human B cell lines, while their functions in NPC have not been investigated. Details of the dysregulated miRNAs and their corresponding targets in NPC are presented in Fig. 1.
Fig. 1.
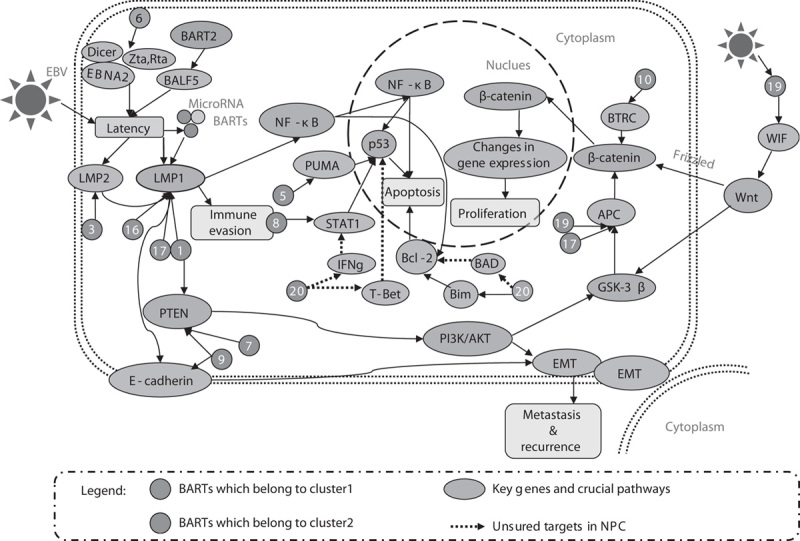
Dysregulated miRNAs and their corresponding targets in nasopharyngeal carcinoma. EBV, Epstein–Barr virus; NPC, nasopharyngeal carcinoma.
BART miRNAs serve as a biomarker
Increasing evidence suggests that circulating microRNAs in malignant diseases are significant, and this is currently a very active field. Several ebv-miRNAs including miR-BART1, miR-BART4, miR-BART7, miR-BART9, miR-BART12, miR-BART13, and miR-BART16 were found to be secreted selectively through two main carriers, microvesicles and exosomes, and transported to adjacent cells. For example, mir-BART7 and mir-BART13 can be detected in plasma from NPC patients; in particular, plasma levels of mir-BART7 and mir-BART13 decrease after radiotherapy (Kawano et al., 2013; Zhang et al., 2015). In addition, BART7 can be used to differentiate patients with NPC from healthy individuals (Chan et al., 2012). On the basis of the above findings, investigators suggest that circulating EBV BART miRNA (detected in plasma or serum) could serve as a biomarker for diagnosis and prognosis in NPC, especially in cases where detection of circulating EBV DNA is not applicable. Another study supports this point. Circulating mir-BART17 appears abundantly in plasma samples from NPC patients irrespective of patient origins. As a marker of NPC plasma samples, ebv-miR-BART17 has good sensitivity (77%) and high specificity (90%) (Gourzones et al., 2013). One remarkable characteristic of circulating microRNAs is their stability. Interestingly, Gourzones et al. (2013) found a marked increase in the concentration of mir-BART17 accompanied by an increase in tumor mass in one patient, suggesting that the concentration of mir-BART17 in plasma may be associated with the progression of tumor. Although it has been speculated that circulating ebv-miRNAs may function as a biomarker, their role in NPC development remains to be understood.
Discussion
EBV infection is a strong predisposing factor in the development of NPC. It is the first human virus that has been found to express miRNAs, most of whose functions remain largely unknown. It can regulate both host cells and virus cells at the transcriptional or the post-transcriptional level by forming imperfect or perfect complementary duplexes with their target mRNAs. Although several studies have reported the upregulation of multiple EBV-encoded miRNAs in NPC, further study is needed to determine the role of mir-BARTs in NPC. For example, BART16, BART11-5p, BART13, and BART15 can target TOM22 (Dolken et al., 2010), EBF1 (Ross et al., 2013), MDS (Borze et al., 2011), and NLRP3 (Haneklaus et al., 2012), respectively, regulating the circumstance of malignancies and modulating the development of cancer; their role in NPC, however, has not been determined. In conclusion, NPC with EBV infection has the propensity to escape immune recognition, induce cell invasion and metastasis, and promote tumorigenesis. mir-BARTs proteins play a restricted role in NPC, whereas BART miRNAs may cooperate with host genes and play a critical role in the development of NPC (Pfeffer and Voinnet, 2006; Riley et al., 2012). To date, at least 25 EBV-miR precursors containing 48 mature miRNAs have been identified; a major challenge is to confirm miRNAs that target more than one gene from EBV or the host cell, ensuring perfect profiling of ebv-miRNAs; in particular, their function in different cancers is difficult to determine and further study is needed.
Acknowledgements
The authors are grateful for the support provided by the National Natural Science Fund (No. 81273595, 81522048, 81573511, 81472802) and the National High Technology Research and Development Program (No. 2012AA02A518, 2012AA02A517).
Conflicts of interest
There are no conflicts of interest.
References
- Barth S, Pfuhl T, Mamiani A, Ehses C, Roemer K, Kremmer E, et al. (2008). Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res 36:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borze I, Scheinin I, Siitonen S, Elonen E, Juvonen E, Knuutila S. (2011). miRNA expression profiles in myelodysplastic syndromes reveal Epstein-Barr virus miR-BART13 dysregulation. Leuk Lymphoma 52:1567–1573. [DOI] [PubMed] [Google Scholar]
- Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF, Yuan CC, et al. (2015a). EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene 34:2156–2166. [DOI] [PubMed] [Google Scholar]
- Cai L, Ye Y, Jiang Q, Chen Y, Lyu X, Li J, et al. (2015b). Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat Commun 6:7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X, et al. (2015c). Gold nano-particles (AuNPs) carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive nasopharyngeal carcinoma. Oncotarget 6:7838–7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Gao W, Ho WK, Wei WI, Wong TS. (2012). Overexpression of Epstein-Barr virus-encoded microRNA-BART7 in undifferentiated nasopharyngeal carcinoma. Anticancer Res 32:3201–3210. [PubMed] [Google Scholar]
- Chan JY, Wong ST, Wei WI. (2015). The role of Epstein-Barr virus-encoded microRNA BART7 status of resection margins in the prediction of local recurrence after salvage nasopharyngectomy for recurrent nasopharyngeal carcinoma. Cancer 121:2358–2366. [DOI] [PubMed] [Google Scholar]
- Chou J, Lin YC, Kim J, You L, Xu Z, He B, Jablons DM. (2008). Nasopharyngeal carcinoma – review of the molecular mechanisms of tumorigenesis. Head Neck 30:946–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, et al. (2008). An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med 205:2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson CW, Port RJ, Young LS. (2012). The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 22:144–153. [DOI] [PubMed] [Google Scholar]
- Dölken L, Malterer G, Erhard F, Kothe S, Friedel CC, Suffert G, et al. (2010). Systematic analysis of viral and cellular microRNA targets in cells latently infected with human gamma-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe 7:324–334. [DOI] [PubMed] [Google Scholar]
- Gourzones C, Ferrand FR, Amiel C, Vérillaud B, Barat A, Guérin M, et al. (2013). Consistent high concentration of the viral microRNA BART17 in plasma samples from nasopharyngeal carcinoma patients – evidence of non-exosomal transport. Virol J 10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu AD, Zeng MS, Qian CN. (2012). The criteria to confirm the role of Epstein-Barr virus in nasopharyngeal carcinoma initiation. Int J Mol Sci 13:13737–13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, et al. (2012). Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol 189:3795–3799. [DOI] [PubMed] [Google Scholar]
- Hikisz P, Kiliańska ZM. (2012). PUMA, a critical mediator of cell death – one decade on from its discovery. Cell Mol Biol Lett 17:646–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CY, Yi YH, Chang KP, Chang YS, Chen SJ, Chen HC. (2014). The Epstein-Barr virus-encoded microRNA MiR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog 10:e1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WT, Lin CW. (2014). EBV-encoded miR-BART20-5p and miR-BART8 inhibit the IFN-γ-STAT1 pathway associated with disease progression in nasal NK-cell lymphoma. Am J Pathol 184:1185–1197. [DOI] [PubMed] [Google Scholar]
- Iizasa H, Wulff BE, Alla NR, Maragkakis M, Megraw M, Hatzigeorgiou A, et al. (2010). Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem 285:33358–33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang QP, Liu SY, He XF, Peng J, Xiong HZ, Xiong ZT, Yang YX. (2011). Relationship between MAP3K5 and Epstein-Barr virus-encoded miR-BART22 expression in nasopharyngeal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 31:1146–1149. [PubMed] [Google Scholar]
- Jung YJ, Choi H, Kim H, Lee SK. (2014). MicroRNA miR-BART20-5p stabilizes Epstein-Barr virus latency by directly targeting BZLF1 and BRLF1. J Virol 88:9027–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Iwata S, Kawada J, Gotoh K, Suzuki M, Torii Y, et al. (2013). Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein-Barr virus infection. J Infect Dis 208:771–779. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi H, Lee SK. (2015). Epstein-Barr virus miR-BART20-5p regulates cell proliferation and apoptosis by targeting BAD. Cancer Lett 356 (Pt B):733–742. [DOI] [PubMed] [Google Scholar]
- Kuzembayeva M, Hayes M, Sugden B. (2014). Multiple functions are mediated by the miRNAs of Epstein-Barr virus. Curr Opin Virol 7:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Yuen KS, Xu R, Tsao SW, Chen HL, Li MF, et al. (2013a). Targeting of DICE1 tumor suppressor by Epstein-Barr virus-encoded miR-BART3* microRNA in nasopharyngeal carcinoma. Int J Cancer 133:79–88. [DOI] [PubMed] [Google Scholar]
- Lei T, Yuen KS, Tsao SW, Chen H, Kok KH, Jin DY. (2013b). Perturbation of biogenesis and targeting of Epstein-Barr virus-encoded miR-BART3 microRNA by adenosine-to-inosine editing. J Gen Virol 94 (Pt 12):2739–2744. [DOI] [PubMed] [Google Scholar]
- Lin TC, Liu TY, Hsu SM, Lin CW. (2013). Epstein-Barr virus-encoded miR-BART20-5p inhibits T-bet translation with secondary suppression of p53 in invasive nasal NK/T-cell lymphoma. Am J Pathol 182:1865–1875. [DOI] [PubMed] [Google Scholar]
- Lo AK, To KF, Lo KW, Lung RW, Hui JW, Liao G, Hayward SD. (2007). Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA 104:16164–16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, Dawson CW, Jin DY, Lo KW. (2012). The pathological roles of BART miRNAs in nasopharyngeal carcinoma. J Pathol 227:392–403. [DOI] [PubMed] [Google Scholar]
- Lung RW, Tong JH, Sung YM, Leung PS, Ng DC, Chau SL, et al. (2009). Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia 11:1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Raab-Traub N. (2012). The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol 22:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Nam CS, Raab-Traub N. (2011). The Epstein-Barr virus BART microRNAs target the proapoptotic protein Bim. Virology 412:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. (2009). Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe 5:376–385. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Voinnet O. (2006). Viruses, microRNAs and cancer. Oncogene 25:6211–6219. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N. (2002). Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol 12:431–441. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R, Donahue H, Garcia D, Tan J, Shimizu N, Rice AP, Ling PD. (2011). Epstein-Barr virus BART9 miRNA modulates LMP1 levels and affects growth rate of nasal NK T cell lymphomas. PLoS One 6:e27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KJ, Rabinowitz GS, Yario TA, Luna JM, Darnell RB, Steitz JA. (2012). EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. EMBO J 31:2207–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross N, Gandhi MK, Nourse JP. (2013). The Epstein-Barr virus microRNA BART11-5p targets the early B-cell transcription factor EBF1. Am J Blood Res 3:210–224. [PMC free article] [PubMed] [Google Scholar]
- Tsang CM, Tsao SW. (2015). The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol Sin 30:107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao SW, Tsang CM, To KF, Lo KW. (2015). The role of Epstein-Barr virus in epithelial malignancies. J Pathol 235:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulalamba W, Janvilisri T. (2012). Nasopharyngeal carcinoma signaling pathway: an update on molecular biomarkers. Int J Cell Biol 2012:594681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y, et al. (2015). Oxidative modification of miR-184 enables it to target Bcl-xL and Bcl-w. Mol Cell 59:50–61. [DOI] [PubMed] [Google Scholar]
- Webb N, Connolly G, Tellam J, Yap AS, Khanna R. (2008). Epstein-Barr virus associated modulation of Wnt pathway is not dependent on latent membrane protein-1. PLoS One 3:e3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AMG, Kong KL, Tsang JWH, Kwong DLW, Guan XY. (2012). Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer 118:698–710. [DOI] [PubMed] [Google Scholar]
- Wong TS, Gao W, Chan JY. (2014). Interactions between E-cadherin and microRNA deregulation in head and neck cancers: the potential interplay. Biomed Res Int 2014:126038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Long ZF, He XS. (2013). Involvement of EBV-encoded BART-miRNAs and dysregulated cellular miRNAs in nasopharyngeal carcinoma genesis. Asian Pac J Cancer Prev 14:5637–5644. [DOI] [PubMed] [Google Scholar]
- Ye Y, Zhou Y, Zhang L, Chen Y, Lyu X, Cai L, et al. (2013). EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochem Biophys Res Commun 436:19–24. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Huang H, Huang L, Sun M, Yan Q, Song Y, et al. (2014). Regulation network and expression profiles of Epstein-Barr virus-encoded microRNAs and their potential target host genes in nasopharyngeal carcinomas. Sci China Life Sci 57:315–326. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zong J, Lin S, Verhoeven RJ, Tong S, Chen Y, et al. (2015). Circulating Epstein-Barr virus microRNAs miR-BART7 and miR-BART13 as biomarkers for nasopharyngeal carcinoma diagnosis and treatment. Int J Cancer 136:E301–E312. [DOI] [PubMed] [Google Scholar]


