Abstract
Radiation-induced lung injury (RILI) is a common complication of thoracic radiotherapy, but efficacious therapy for RILI is lacking. This study ascertained whether glycyrrhetinic acid (GA; a functional hydrolyzed product of glycyrrhizic acid, which is extracted from herb licorice) can protect against RILI and investigated its relationship to the transforming growth factor (TGF)-β1/Smads signaling pathway. C57BL/6 mice were divided into four groups: a control group, a GA group and two irradiation (IR) groups. IR groups were exposed to a single fraction of X-rays (12 Gy) to the thorax and administered normal saline (IR + NS group) or GA (IR + GA group). Two days and 17 days after irradiation, histologic analyses were performed to assess the degree of lung injury, and the expression of TGF-β1, Smad2, Smad3 and Smad7 was recorded. GA administration mitigated the histologic changes of lung injury 2 days and 17 days after irradiation. Protein and mRNA expression of TGF-β1, Smad2 and Smad3, and the mRNA level of Smad7, in lung tissue were significantly elevated after irradiation. GA decreased expression of TGF-β1, Smad2 and Smad3 in lung tissue, but did not increase Smad7 expression. GA can protect against early-stage RILI. This protective effect may be associated with inhibition of the TGF-β1/Smads signaling pathway.
Keywords: glycyrrhetinic acid, thoracic irradiation, pneumonia, TGF-β1, Smad
INTRODUCTION
Radiation-induced lung injury (RILI) is a common complication in radiotherapy inpatients with thoracic tumors [1]. It can be separated into two stages: acute/subacute pneumonia (early stage) and pulmonary fibrosis (late stage). The symptoms of RILI are a cough, asthma, and even respiratory failure. It affects quality of life greatly, and may even lead to death [2].
Drugs to treat radiation-induced pneumonia and radiation-induced pulmonary fibrosis are inadequate. Corticosteroids are the most common therapy for radiation-induced symptomatic pneumonia. Corticosteroids can relieve some symptoms, but cannot prevent the development of RILI [3, 4]. The anti-inflammatory agent celecoxib has been considered for use against chronic lung damage [5], but clinical evidence for its efficacy is lacking. Amifostine has been approved by the U.S. Food and Drug Administration for use as an anti–free radical drug. It has elicited some protective effects against radiation-induced pneumonia [6] but not fibrosis. In addition, the necessity for intravenous injection 30 min before radiation exposure and severe side effects make long-term use for chronic lung fibrosis impossible. Hence, finding effective therapy for RILI with few and non-severe side effects is urgent [7–9].
Glycyrrhetinic acid (GA) is a functional hydrolyzed product of glycyrrhizic acid, which is extracted from herb licorice. In several countries, glycyrrhizic acid is used as a sweetener or flavoring in confectionery, pharmaceuticals, and tobacco products [10, 11]. It can be taken orally and has low toxicity. It has also been used for the treatment of dermatitis [12], chronic hepatitis [13], cough, asthma and pneumonia [14]. Several studies have shown that GA can alleviate inflammatory lung disease [15–18]. Inflammation is the predominant histologic and physiologic feature of early-phase RILI [19, 20]. Hence, this study investigated whether GA can protect against RILI and we have discussed the potential mechanism for this effect.
MATERIALS AND METHODS
Animals
The study was approved by the ethics committee of our institution. Eight-week-old female specific-pathogen-free C57BL/6 mice with weight of 19–22 g (Shanghai SLRC Laboratory Animals, Shanghai, China) were divided randomly into four groups: normal control (n = 19); GA without irradiation (IR) (GA group; n = 20); IR plus normal (0.9%) saline (IR + NS group; n = 20); and IR plus GA (IR + GA group; n = 20). Mice were housed in conventional cages under a 12 h–12 h light–dark cycle and controlled temperature and humidity. Sterilized food and water were supplied for the mice.
Radiation procedure
Anesthesia (10 ml/kg 4% chloral hydrate, i.p.) was induced in the mice. Each mouse was placed in an organic glass cage (made by the research team) with its neck fixed and body stretched fully. Groups of 7 or 8 mice were placed on an IR bed with their necks in a straight line and irradiated together. A linear accelerator (Clinac 600 C/D; Varian Medical Systems, Palo Alto, CA, USA) created an X-ray IR field of 38 × 1.2 cm2 to encompass the entire thorax of each mouse on the bed (voltage, 6 mV; direction, 0°; total dose, 12 Gy; rate, 3 Gy/min; distance from source to skin, 100 cm). Organs outside the IR field were shielded by the multileaf collimator of the linear accelerator.
Drug administration
Two IR groups and the GA group were treated with the corresponding agents by gavage once a day from 2 h before IR to 7 days after IR, then every 2 days until the 17th day after IR. Mice in the IR + NS group were administered 10 ml/kg NS each time, and mice in the IR + GA and GA groups were administered 40 mg/kg GA (Acros Organics, Pittsburgh, PA, USA) each time.
Histologic examination
Two days after IR, 10 mice from each of the four groups were euthanized, and the remaining mice were euthanized on Day 17. The left upper lobes of the lungs were fixed in 10% neutral-buffered formalin overnight, embedded in paraffin, and cut into sections (thickness, 5 μm). Some sections were stained with hematoxylin and eosin (H&E) for histopathologic analyses and scored in a blinded manner according to a semi-quantitative scale [21]. Five random fields (magnification ×200 for each section) were scored by two clinicians using an optical microscope. The following system was used to score histologic changes in pneumonia: 1, no pneumonia; 2, pneumonia alterations in 1–20% of the field; 3, pneumonia alterations in 21–50% of the field; 4, pneumonia alterations in 51–100% of the field. Some other sections were used for immunohistochemical analyses (see below).
Protein extraction and enzyme-linked immunosorbent assay
The protein in the right lungs was extracted using a Protein Extraction kit (Shanghai Sangon Biotechnology, Shanghai, China). Protein concentrations were determined using a Bicinchoninic Acid Protein Assay kit (Shanghai Sangon Biotechnology). Concentrations of transforming growth factor (TGF)-β1 in lung homogenates were quantified with a Mouse TGF-β1 protein extraction and enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), following the manufacturer's instructions. Optical density values were detected using a Microplate Reader (iMark; Bio-Rad, Hercules, CA, USA) at 450 nm.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from the left lower lobes of the lungs of the mice in each group using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). RNase-free DNase (Invitrogen) was used to remove contaminating genomic DNA according to the manufacturer's instructions. cDNA was synthesized from the total RNA using a Reverse Transcription system (Promega, Fitchburg, WI, USA), following the manufacturer's protocols. RNA extraction and quantitative real-time polymerase chain reactions (qRT-PCRs) were carried out in a total volume of 20 μl using an ABI7500 RT-PCR system (Applied Biosystems, Foster City, CA, USA) with GoTaq® qPCR Master Mix (Promega) under the following conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, then each of 95°C, 15°C and 95°C for 15 s mRNA levels of TGF-β1, Smad2, Smad3 and Smad7 in lung tissue were determined for each group with the mRNA level of β-actin used for standardization. Sequences of mouse primers are listed in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | Primer | Sequence |
|---|---|---|
| TGF-β1 | forward | 5′-TTTGGAGCCTGGACACACAGTA-3′ |
| reverse | 5′-CGTAGTAGACGATGGGCAGTG-3′ | |
| Smad2 | forward | 5′-CGGCTGAACTGTCTCCTACTAC-3′ |
| reverse | 5′-TATGCGATTGAACACCAGAATG-3′ | |
| Smad3 | forward | 5′-AGGTGTGCGGCTCTACTACATC-3′ |
| reverse | 5′-GTTGGGAGACTGGACGAAAATA-3′ | |
| Smad7 | forward | 5′- AGGCTGTGTTGCTGTGAATCT-3′ |
| reverse | 5′- CTCCAGAAGAAGTTGGGAATCT-3′ | |
| β-actin | forward | 5′-GAAGGACTCCTATGTGGGTGAC-3′ |
| reverse | 5′-TTCACGGTTGGCCTTAGG-3′ |
Immunohistochemical analyses
The remaining sections of left upper lung lobes were deparaffinized with xylene and hydrated with ethanol of different concentration gradients. These actions were followed by antigen retrieval with microwave thermal remediation in 0.01 M citrate buffer (pH 6.0; for Smad2 staining) or TRIS/ethylenediamine tetra-acetic acid buffer (pH 9.0; for TGF-β1 or Smad3 staining). Endogenous peroxide activity was inactivated by incubating samples in 3% hydrogen peroxide for 10 min. Sections were incubated with antibodies against TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Smad2 (Abcam, Cambridge, MA, USA) and Smad3 (Abcam) at 1:100 dilution at room temperature for 2 h, and the Polink-2 Plus® Polymer Horseradish Peroxidase Detection system for rabbit primary antibody (GBI Laboratories, Mukilteo, WA, USA) according to the manufacturer's instructions. A 3,3’-Diaminobenzidine kit (ZSGB-BIO, Beijing, China) was used for signal amplification, and hematoxylin was used as the counterstain. Appropriate positive and negative controls of relevant histologic tissue sections were included for each stain to ensure signal specificity. Stained sections were analyzed in a blinded manner according to the method of Remmele and Stegner [22]. Each section was observed randomly in five fields of magnification (×400) using a system to score staining intensity (scored as A) and percentage of positive cells (scored as B). That is, for A: 0, not stained; 1, light-yellow stain; 2, pale-brown stain; 3, dark-brown stain. Then, for B: 0, no positive cells; 1, fewer than one-third of cells stained; 2, one-third to two-thirds of cells stained; 3, more than two-thirds of cells stained. Scores of each section were the average of five fields (calculated by multiplying A and B).
Statistical analyses
Data are the mean ± standard deviation (SD). Statistical analyses were carried out using one-way ANOVA, followed by Bonferroni's multiple comparison test. P < 0.05 was considered significant.
RESULTS
Histologic evaluation of lung injury
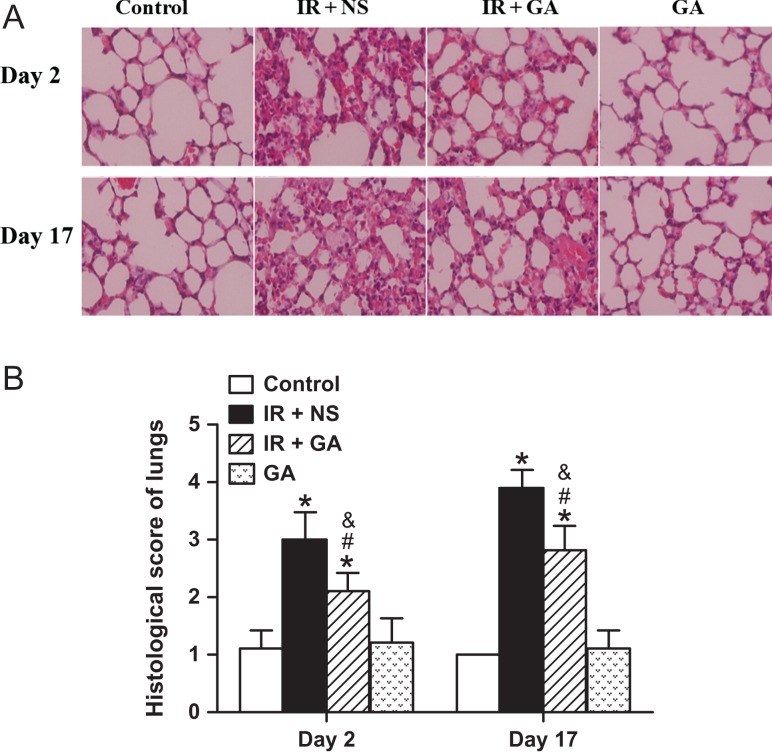
Two clinicians evaluated H&E-stained sections of lungs and scored histologic changes after IR. The control group and the GA group both showed no obvious inflammatory changes. However, massive infiltration of inflammatory cells in the alveolae, and alveolar septal edema were observed in the IR + NS group, whereas fewer inflammatory cells and less edema was seen in the IR + GA group (Fig. 1A). Histologic scoring demonstrated that lungs irradiated with a single fraction of 12 Gy had significant changes due to IR-induced injury on Day 2 and Day 17, and that GA administration significantly mitigated IR-induced injury to the lungs as compared with NS administration at each time-point (P < 0.001, Fig. 1B).
Fig. 1.
Histologic detection and semi-quantification of radiation-induced lung injury in mice. Mice of the IR + NS group and IR + GA group were exposed to a single fraction of X-ray irradiation (12 Gy) to the thorax and administered NS or GA. The GA group was not exposed to irradiation. Panel A: Typical hematoxylin and eosin (H&E)-stained sections of mouse lungs from each group 2 days and 17 days after irradiation (magnification × 400). Panel B: Histologic scores of lung injury from H&E-stained sections. Data are the mean ± SD. *P < 0.05, vs control group; #P < 0.05, vs IR + NS group; &P < 0.05, vs GA group, at identical time-points, by one-way ANOVA (followed by Bonferroni's multiple comparison test). IR = irradiation, NS = normal saline, GA = glycyrrhetinic acid.
Protein expression of TGF-β1 in lung tissue
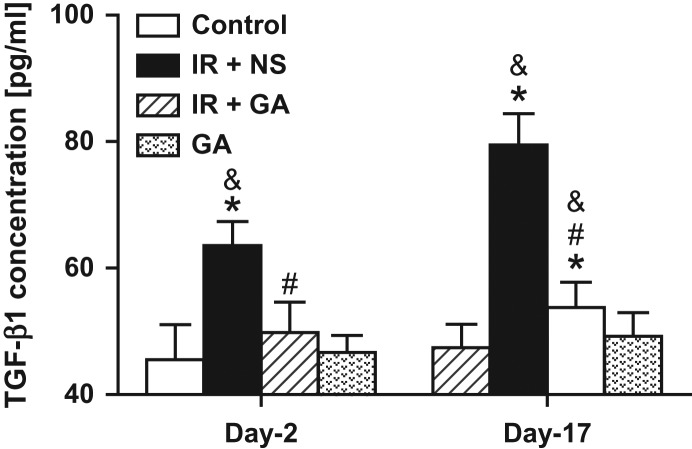
TGF-β1 concentrations (as determined by ELISA) of lung homogenates from irradiated mice administered NS (Day 2, 63.56 ± 3.81; Day 17, 79.48 ± 4.96 pg/ml) were significantly higher than those from mice in the control group (45.51 ± 5.58; 47.41 ± 3.73 pg/ml, respectively) and those from mice in the GA group (46.66 ± 2.72; 49.22 ± 3.74 pg/ml, respectively) (P < 0.05). GA administration had no significant effect on TGF-β1 concentrations of lung homogenates from non-IR mice compared with those from the normal control group on either Day 2 or Day 17 (P < 0.05). GA administration significantly reduced TGF-β1 expression in irradiated lungs at both time-points (P < 0.05). TGF-β1 concentrations in lung tissues of mice from the IR + GA group (Day 2, 49.82 ± 4.81; Day 17, 53.78 ± 3.93 pg/ml) were not significantly different from those of the control group or the GA group on Day 2 (P > 0.05), but were significantly different from those on Day 17 (P < 0.05, Fig. 2).
Fig. 2.
ELISA detection of TGF-β1 concentrations in mouse lungs after irradiation. Mice of two irradiated groups, exposed to a single fraction of X-ray irradiation (12 Gy) to the thorax, were treated with NS or GA. The GA group was not exposed to irradiation. Concentrations of the cytokine TGF-β1 in lung homogenates were determined by ELISA 2 days and 17 days after irradiation. Data are the mean ± SD.*P < 0.05, vs control group; #P < 0.05, vs IR + NS group; &P < 0.05, vs GA group, at identical time-points, by one-way ANOVA (followed by Bonferroni's multiple comparison test). IR = irradiation, NS = normal saline, GA = glycyrrhetinic acid.
Gene expression of TGF-β1, Smad2, Smad3 and Smad7 in lung tissue
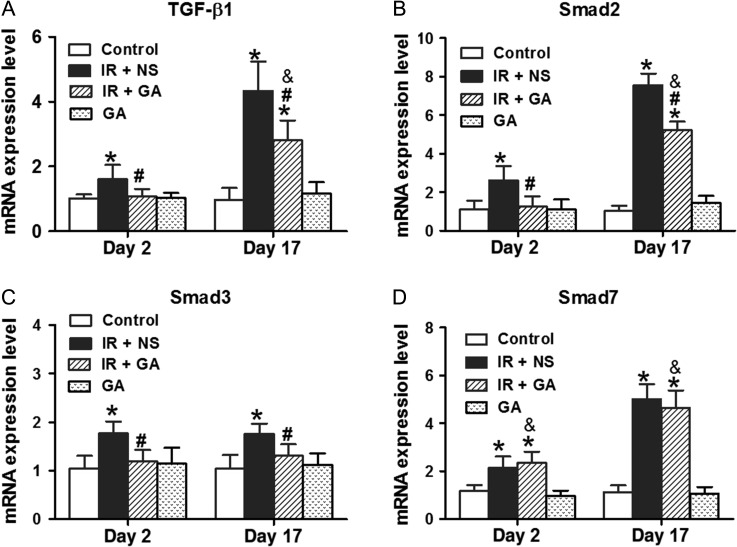
Gene expression of TGF-β1, Smad2, Smad3 and Smad7 in lung tissues was determined by qRT-PCR (Fig. 3A–D). IR to the thorax with a single fraction of 12 Gy significantly upregulated the gene expression of TGF-β1, Smad2, Smad3 and Smad7 in lungs on Day 2 and Day 17 (P < 0.05); the gene expression of TGF-β1, Smad2, Smad3 and Smad7 for non-irradiated lungs of mice with GA administration was not significantly different from those with NS administration. Irradiated lungs from GA-administered mice had significantly lower gene levels of TGF-β1, Smad2 and Smad3 on Day 2 and Day 17 compared with those from NS-administered mice (P < 0.05). However, expression of the Smad7 gene for irradiated lungs was not significantly different between GA-administered and NS-administered mice.
Fig. 3.
qRT-PCR analyses of gene expression in mouse lungs after irradiation. Mice were irradiated with a single fraction of 12 Gy and treated with NS or GA (except for the control group and GA group). mRNA levels of TGF-β1 (panel A), Smad2 (panel B), Smad3 (panel C) and Smad7 (panel D) were analyzed by qRT-PCR 2 days and 17 days after irradiation. Data are the mean ± SD. *P < 0.05, vs control group; #P < 0.05, vs IR + NS group; &P < 0.05, vs GA group, at identical time-points, by one-way ANOVA (followed by Bonferroni's multiple comparison test). IR = irradiation, NS = normal saline, GA = glycyrrhetinic acid.
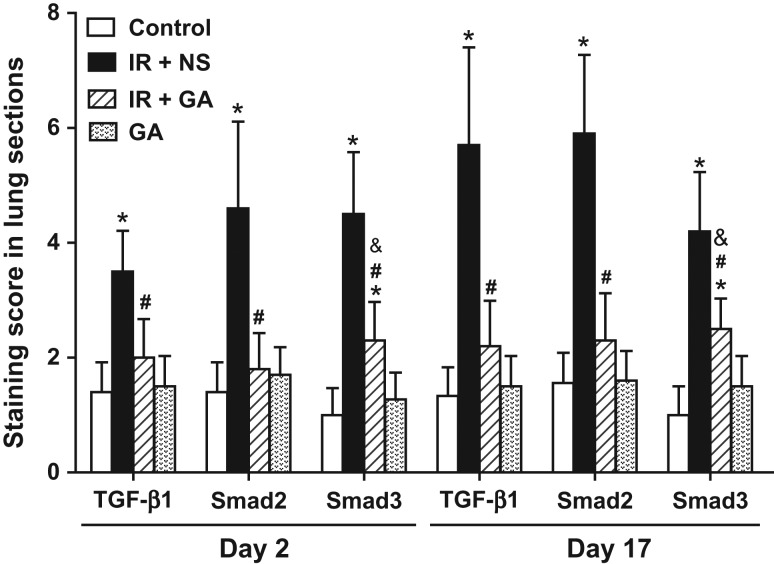
Immunohistochemical examination of expression of TGF-β1, Smad2 and Smad3
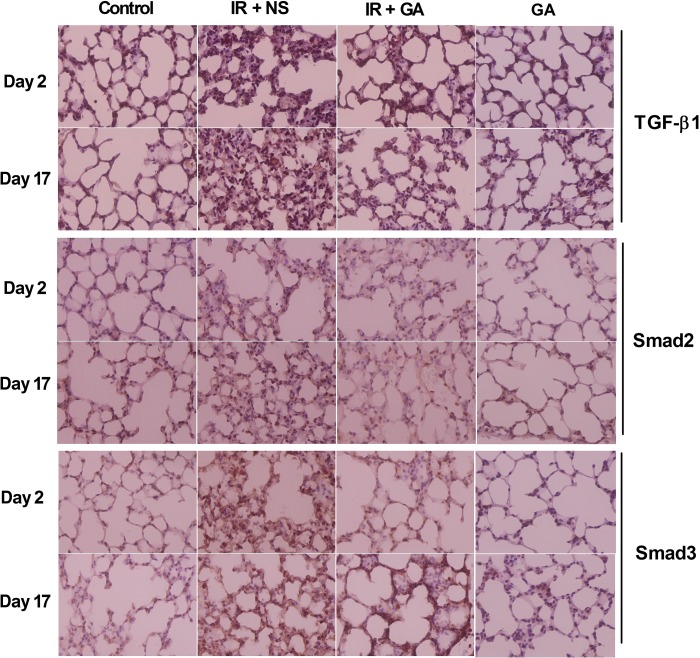
Expression of TGF-β1, Smad2 and Smad3 in lung sections 2 days and 17 days after IR exposure was scored. Lung sections of irradiated groups at both time-points had higher staining intensities and more positive cells for TGF-β1, Smad2 and Smad3 as compared with the non-irradiated control group (Fig. 4A–C). GA administration significantly reduced the expression of TGF-β1, Smad2 and Smad3 in irradiated lung tissue compared with NS administration on Day 2 and Day 17 (P < 0.05), but the levels were not significantly changed in non-irradiated lung tissue on Day 2 and Day 17. Importantly, levels of TGF-β1 and Smad2 protein in lungs from irradiated mice administered GA were not significantly different to their baseline levels on Day 2 and Day 17 (P > 0.05) (Fig. 5).
Fig. 4.
Immunohistochemical staining of mouse lung tissue after thoracic irradiation. Mice in group IR + NS and IR + GA were exposed to a single fraction of 12 Gy and administered NS or GA. Mice in group GA were not irradiated. Typical TGF-β1-, Smad2- or Smad3-stained sections of mouse lungs from each group 2 days and 17 days after irradiation (×400 magnification). IR = irradiation, NS = normal saline, GA = glycyrrhetinic acid.
Fig. 5.
Semi-quatitative analyses of immunohistochemical detection in mouse lungs after irradiation. Mice in the irradiation groups were exposed to a single fraction of 12 Gy and treated with NS or GA. Protein levels of TGF-β1, Smad2 and Smad3 in mouse lungs were measured by immunohistochemical detection and analyzed semi-quantitatively with scoring 2 days and 17 days after irradiation. Data are the mean ± SD. *P < 0.05, vs control group; #P < 0.05, vs IR + NS group; &P < 0.05, vs GA group, at identical time-points, by one-way ANOVA (followed by Bonferroni's multiple comparison test). IR = irradiation, NS = normal saline, GA = glycyrrhetinic acid.
DISCUSSION
The present study showed that GA can protect mice against early-phase lung injury and decrease TGF-β1 concentrations in the lung after thoracic IR. Also, thoracic IR significantly elevated expression of the mRNAs and proteins of TGF-β1, Smad2 and Smad3, as well as the mRNA level of Smad7 in lung tissue. GA decreased the upregulated expression of mRNA and protein of TGF-β1, Smad2 and Smad3 in lung tissue, but did not increase the mRNA expression of Smad7.
Recruitment of inflammatory cells into the lung and increased production of inflammatory cytokines are the main features of RILI in the acute phase [23–26]. Evidence suggests a central role for inflammation in the initiation and establishment of RILI [27]. Studies have shown that glycyrrhizin and GA can downregulate expression of inflammatory mediators in vitro [28, 29], and that they could be agents for the treatment of inflammatory-mediated lung disease [28]. Some experiments in vivo have shown that glycyrrhizic acid and GA alleviate inflammatory lung disease [18, 30]. After ingestion, glycyrrhizic acid is hydrolyzed to GA upon encountering gastric acid (pH 1.5–2) or disaccharidase released from the intestine or bacteria, so only GA can reach the circulation and exerts its effects. Therefore, this research team studied whether oral administration of GA can protect against the lung injury induced by radiation in the acute phase. Histopathologic analyses showed that GA reduced the lung injury induced by radiation. Some studies have also demonstrated the radioprotection afforded by licorice extracts on other tissues. Gandhi et al. found that glycyrrhizae and glycyrrhizic acid enhanced recovery of the spleen, thymus and testes from the injury induced by gamma-ray IR in mice [31]. Lin et al. reported that glycyrrhizic acid protected the DNA of the leukocytes of peripheral blood and bone marrow cells in mice from radiation-induced strand breaks [32]. Chandrasekharan et al. demonstrated that glycyrrhizic acid, silver nanoparticles and their complexes protected the hematopoietic and gastrointestinal systems from radiation, and offered preferential radioprotection to normal cells in tumor-bearing animals [33].
How GA protects against RILI needs to be investigated. Recent studies have revealed some aspects of the underlying mechanism of RILI, but the exact pathogenesis is not known. It is considered that TGF-β1 (a cytokine that can regulate the growth and differentiation of cells) plays an important part in radiation-induced tissue damage (especially in radiation-induced pneumonia and pulmonary fibrosis) [34–36]. TGF-β1 signals through cell-surface serine/threonine kinase receptors to activate Smad proteins, which modulate the transcription of target genes [37]. Some studies have demonstrated that the TGF-β1/Smad signaling pathway has an essential role in RILI [38–40]. In the present study, the research team found some change in TGF-β1 expression in the lung after radiation exposure. Therefore, this study investigated the role of the TGF-β1/Smads signaling pathway in the protection afforded against radiation pneumonia by GA.
This study revealed that the mRNA and protein expression of TGF-β1 in lung tissue was elevated at the early stage of RILI, and that GA downregulated expression of TGF-β1. C57BL/6 mice also showed similar results after thoracic IR with a single dose of 12 Gy or 12.5 Gy; the TGF-β1 level in lung tissue increased between 12 h and 8 weeks after IR [41, 42]. Of the Smads, Smad2 and Smad3 mediate receptor-activated signals, whereas Smad7 mediates inhibitory signals [37]. At the early stage of RILI, the research team observed increasing expression of not only the mediators of signal activation (Smad2 and Smad3), but also that of the mediator of signal inhibitor, Smad7. A possible explanation could be that the pro-inflammatory cytokine TNF-α, which was elevated in the bronchoalveolar lavage fluid of mice after thoracic IR, increased expression of Smad7 by activating nuclear factor-kappa B [43, 44]. Importantly, reduction of the expression of Smad2 and Smad3, but not increase in the expression of Smad7, by GA was revealed. Our results suggest that the protection afforded by GA against IR pneumonia is related to inhibition of activation of the TGF-β1/Smads signaling pathway, which has a role in RILI.
Further studies are needed to investigate the effect of GA against lethal whole-body IR-induced mortality and ascertain the relationship between the radioprotective effect of GA in vivo. Investigating the dose–effect relationship and protective effect of GA against radiation-induced pulmonary fibrosis will be important.
CONCLUSION
Our results demonstrated that GA can alleviate radiation-induced pneumonia, and that this may be associated with inhibition of activation of the TGF-β1/Smads signaling pathway. This research group hypothesizes that GA may be useful for the treatment of RILI.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by Grants from the Natural Science Foundation of Fujian Province of China (Grant No. 2012J01338 and No. 2014J01308), Medical Innovation Research of Fujian Province of China (Grant No. 2012-CX-20), Youth Scientific Research of the Health Department of Fujian Province of China (Grant No. 2013-1 -30), Department of Science and Technology of Fujian Province of China (Grant No. 2015Y4004) and the Key Laboratory Fund from Fujian Medical University to Fujian Key Laboratory of Individualized Active Immunotherapy and to Key Laboratory of Radiation Biology of Fujian Province University (Grant No. 0000-081919).
REFERENCES
- 1.Carver JR, Shapiro CL, Ng A, et al. . American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991–4008. [DOI] [PubMed] [Google Scholar]
- 2.Shi A, Zhu G, Wu H, et al. . Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non–small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol 2010;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwok E, Chan CK. Corticosteroids and azathioprine do not prevent radiation-induced lung injury. Can Respir J 1998;5:211–4. [DOI] [PubMed] [Google Scholar]
- 4.Takigawa N, Segawa Y, Saeki T, et al. . Bronchiolitis obliterans organizing pneumonia syndrome in breast-conserving therapy for early breast cancer: radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys 2000;48:751–5. [DOI] [PubMed] [Google Scholar]
- 5.Gore E. Celecoxib and radiation therapy in non–small-cell lung cancer. Oncology 2004;18:10–4. [PubMed] [Google Scholar]
- 6.Choi NC. Radioprotective effect of amifostine in radiation pneumonitis. Semin Oncol 2003;30:10–7. [DOI] [PubMed] [Google Scholar]
- 7.Mathew B, Huang Y, Jacobson JR, et al. . Simvastatin attenuates radiation-induced murine lung injury and dysregulated lung gene expression. Am J Respir Cell Mol Biol 2011;44:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmood J, Jelveh S, Calveley V, et al. . Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol 2011;87:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molteni A, Wolfe LF, Ward WF, et al. . Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des 2007;13:1307–16. [DOI] [PubMed] [Google Scholar]
- 10.Størmer FC, Reistad R, Alexander J.. Glycyrrhizic acid in liquorice—evaluation of health hazard. Food Chem Toxicol 1993;31:303–12. [DOI] [PubMed] [Google Scholar]
- 11.Tolstikov GA, Baltina LA, Shul'ts EE, et al. . [Glycyrrhizic acid]. Bioorg Khim 1997;23:691–709. [PubMed] [Google Scholar]
- 12.Paolino D, Lucania G, Mardente D, et al. . Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J Control Release 2005;106:99–110. [DOI] [PubMed] [Google Scholar]
- 13.Muriel P, Rivera-Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol 2008;28:93–103. [DOI] [PubMed] [Google Scholar]
- 14.Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000;120:849–62. [DOI] [PubMed] [Google Scholar]
- 15.Kao TC, Shyu MH, Yen GC.. Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J Agric Food Chem 2010;58:8623–9. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z, Ohtaki Y, Kai K, et al. . Critical roles of platelets in lipopolysaccharide-induced lethality: effects of glycyrrhizin and possible strategy for acute respiratory distress syndrome. Int Immunopharmacol 2005;5:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram A, Mabalirajan U, Das M, et al. . Glycyrrhizin alleviates experimental allergic asthma in mice. Int Immunopharmacol 2006;6:1468–77. [DOI] [PubMed] [Google Scholar]
- 18.Menegazzi M, Di Paola R, Mazzon E, et al. . Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol Res 2008;58:22–31. [DOI] [PubMed] [Google Scholar]
- 19.Travis EL, Down JD, Holmes SJ, et al. . Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat Res 1980;84:133–43. [PubMed] [Google Scholar]
- 20.Szabo S, Ghosh SN, Fish BL, et al. . Cellular inflammatory infiltrate in pneumonitis induced by a single moderate dose of thoracic x radiation in rats. Radiat Res 2010;173:545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szapiel SV, Elson NA, Fulmer JD, et al. . Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis 1979;120:893–9. [DOI] [PubMed] [Google Scholar]
- 22.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe 1987;8:138–40. [PubMed] [Google Scholar]
- 23.Johnston CJ, Williams JP, Elder A, et al. . Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res 2004;30:369–82. [DOI] [PubMed] [Google Scholar]
- 24.Vergara JA, Raymond U, Thet LA. Changes in lung morphology and cell number in radiation pneumonitis and fibrosis: a quantitative ultrastructural study. Int J Radiat Oncol Biol Phys 1987;13:723–32. [DOI] [PubMed] [Google Scholar]
- 25.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem 2009;16:130–43. [DOI] [PubMed] [Google Scholar]
- 26.Johnston CJ, Hernady E, Reed C, et al. . Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiat Res 2010;173:522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am J Respir Crit Care Med 1998;157:S123–9. [DOI] [PubMed] [Google Scholar]
- 28.Matsui S, Matsumoto H, Sonoda Y, et al. . Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol 2004;4:1633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CY, Kao TC, Lo WH, et al. . Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem 2011;59:7726–33. [DOI] [PubMed] [Google Scholar]
- 30.Shi JR, Mao LG, Jiang RA, et al. . Monoammonium glycyrrhizinate inhibited the inflammation of LPS-induced acute lung injury in mice. Int Immunopharmacol 2010;10:1235–41. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi NM, Maurya DK, Salvi V, et al. . Radioprotection of DNA by glycyrrhizic acid through scavenging free radicals. J Radiat Res 2004;45:461–8. [DOI] [PubMed] [Google Scholar]
- 32.Lin IH, Hau DM, Su MJ, et al. . Effects of glycyrrhizae and glycyrrhizic acid on radiation injury in mice. Am J Chin Med 1996;24:279–88. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekharan DK, Nair CK. Studies on silver nanoparticle–glycyrrhizic acid complex as a radioprotector and an adjuvant in radiotherapy under in vivo conditions. Cancer Biother Radiopharm 2012;27:642–51. [DOI] [PubMed] [Google Scholar]
- 34.Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 2000;10:296–307. [DOI] [PubMed] [Google Scholar]
- 35.Rubin P, Johnston CJ, Williams JP, et al. . A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 1995;33:99–109. [DOI] [PubMed] [Google Scholar]
- 36.Haiping Z, Takayama K, Uchino J, et al. . Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther 2006;13:864–72. [DOI] [PubMed] [Google Scholar]
- 37.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–84. [DOI] [PubMed] [Google Scholar]
- 38.Flechsig P, Dadrich M, Bickelhaupt S, et al. . LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 2012;18:3616–27. [DOI] [PubMed] [Google Scholar]
- 39.Anscher MS, Thrasher B, Zgonjanin L, et al. . Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys 2008;71:829–37. [DOI] [PubMed] [Google Scholar]
- 40.Puthawala K, Hadjiangelis N, Jacoby SC, et al. . Inhibition of integrin αvβ 6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 2008;177:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkelstein JN, Johnston CJ, Baggs R, et al. . Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys 1994;28:621–31. [DOI] [PubMed] [Google Scholar]
- 42.Rube CE, Uthe D, Schmid KW, et al. . Dose-dependent induction of transforming growth factor beta (TGF-β) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys 2000;47:1033–42. [DOI] [PubMed] [Google Scholar]
- 43.Hong JH, Jung SM, Tsao TC, et al. . Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol 2003;79:159–67. [DOI] [PubMed] [Google Scholar]
- 44.Bitzer M, von Gersdorff G, Liang D, et al. . A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/RelA. Genes Dev 2000;14:187–97. [PMC free article] [PubMed] [Google Scholar]