Abstract
Context
South Africa has the most HIV infections of any country in the world, yet little is known about the adolescent continuum of care from HIV diagnosis through viral suppression.
Objective
To determine the adolescent HIV continuum of care in South Africa.
Data sources
We searched PubMed, Google Scholar and online conference proceedings from International AIDS Society (IAS), International AIDS Conference (AIDS) and Conference on Retrovirology and Opportunistic Infections (CROI) from 1 January 2005 to 31 July 2015.
Data extraction
We selected published literature containing South African cohorts and epidemiological data reporting primary data for youth (15–24 years of age) at any stage of the HIV continuum of care (ie, diagnosis, treatment, retention, viral suppression). For the meta-analysis we used six sources for retention in care and nine for viral suppression.
Results
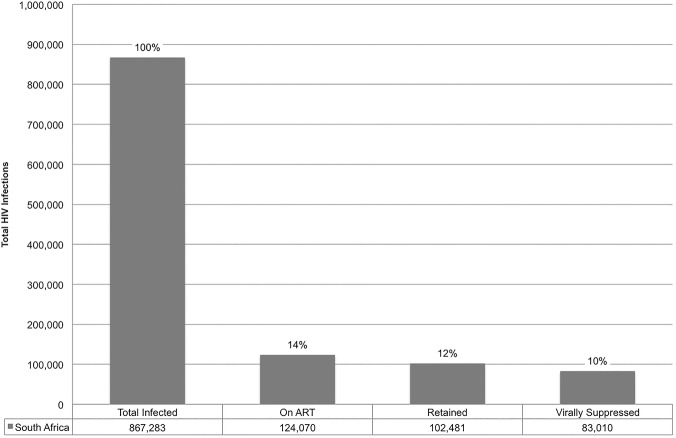
Among the estimated 867 283 HIV-infected youth from 15 to 24 years old in South Africa in 2013, 14% accessed antiretroviral therapy (ART). Of those on therapy, ∼83% were retained in care and 81% were virally suppressed. Overall, we estimate that 10% of HIV-infected youth in South Africa in 2013 were virally suppressed.
Limitations
This analysis relies on published data from large mostly urban South Africa cohorts limiting the generalisability to all adolescents.
Conclusions
Despite a large increase in ART programmes in South Africa that have relatively high retention rates and viral suppression rates among HIV-infected youth, only a small percentage are virally suppressed, largely due to low numbers of adolescents and young adults accessing ART.
Key questions.
What is already known about this topic?
The incidence of HIV infection among adolescents and young adults in South Africa is high.
Perinatally HIV-infected children are ageing to adolescence and young adulthood.
Adolescents and young adults have lower retention in care and lower viral suppression rates than older adults.
What are the new findings?
There are an estimated 720 000 HIV-infected youth aged 15–24 in South Africa.
Overall 10% of HIV-infected youth in South Africa are virally suppressed.
Among the estimated 867 283 HIV-infected youth aged 15–24 years in South Africa in 2013, 14% accessed antiretroviral therapy (ART).
Of those on therapy, approximately 83% were retained in care and 81% were virally suppressed.
Recommendations for policy
Increasing antiretroviral treatment in adolescents and young adults.
Improving adolescent-friendly services to increase retention and viral suppression rates.
Preparation and planning for transition care for HIV-infected youth.
Introduction
South Africa has the highest number of HIV infections of any country in the world with an estimated 6.8 million total infections.1 Access to antiretroviral therapy (ART) did not begin until 2004, a delay that contributed to high numbers of perinatal HIV infections in the late 1990s and early 2000s.2 Even with the implementation of the South African National Antiretroviral Treatment Programme, enrolment was slow due to political, cultural, infrastructure, geographic and economic issues.3 4 Many perinatally HIV-infected infants died during the delays in ART rollout.3 5
With improved access to ART, perinatally HIV-infected infants are surviving into adolescence and early adulthood.2 At the same time, adolescents and young adults, particularly females aged 15–24, have a high incidence of new HIV infections.6 These two groups, perinatally infected and non-perinatally infected adolescents and young adults, make up a significant portion (11%) of South Africa's total number of HIV infections.
Now, with more than 10 years of an established national ART programme and its increased scale up in recent years, South Africa has the largest national ART programme in the world.2 7 Backed by emerging evidence, national ART guidelines support earlier initiation of ART with simpler, more potent and less toxic regimens leading to increased numbers of individuals accessing treatment.8 The scale-up has contributed to high levels of viral suppression, lower mortality rates and decreased HIV transmission among large adult populations in South Africa.2 9–11
Several groups have evaluated the continuum of HIV care (from HIV infection to diagnosis, linkage to care, retention in care, ART initiation and viral suppression) in adults from resource-rich countries. Analyses indicate that only a small number of HIV-infected individuals are fully benefitting from therapy.2 12–14 In sub-Saharan Africa, ∼24% of HIV-infected individuals successfully navigate the continuum of care and are virally suppressed.2 An analysis of the adolescent and young adult continuum of care in the USA estimated that only 6% of HIV-infected youth were virally suppressed.13 The continuum of care for HIV-infected adolescents and young adults in South Africa has not previously been described.
The purpose of this analysis is to assess the number perinatally and non-perinatally HIV-infected adolescents requiring ART and surviving into adulthood. Preparing for this growing population has major implications for South Africa's national treatment programme, including the need for adolescent-friendly HIV prevention and treatment programmes as well as transition to adult services for HIV-infected youth.
Below, we present the results of a systematic review estimating the number of adolescents and young adults infected with HIV in South Africa, the number of those on ART and HIV-related mortality. In addition, we report results from our meta-analysis on the percentage of HIV-infected adolescents in South Africa who are retained in care and virally suppressed.
Methods
We searched PubMed, Google Scholar and online conference proceedings from the International AIDS Society (IAS), the International AIDS Conference (AIDS) and the Conference on Retrovirology and Opportunistic Infections (CROI) from 1 January 2005 to 31 July 2015. Key words and medical subject headings relevant to age (ie, adolescent, adolescence, teen, youth, young adults) were cross-referenced with terms associated with the HIV continuum of care (ie, retention, loss to follow-up, viral suppression, ART, virological failure, outcomes and transition). We sought unpublished data from the South African Department of Health and major academic centres in Johannesburg, Durban, Pietermaritzburg and Cape Town. We reviewed literature in English reporting primary observational data from South African cohorts or epidemiological studies that included specific age ranges, including adolescents and young adults within our target age group of 15–24 years. We attempted to contact the corresponding authors of studies that included ages outside of this range. Because studies reported variable age ranges, we included studies in our analysis that reported primary data on youth, including the ranges of 15–24 years. We report age ranges as described by the original authors; therefore, this analysis includes adolescents and young adults as young as 9 years old and as old as 29 years old due to different binning of age groups by original authors.
We used epidemiological data from South African HIV antenatal HIV prevalence rates, reported number of live births and estimated mother-to-child HIV transmission rates to estimate the annual number of HIV-infected infants born each year in South Africa.
In our meta-analysis, we calculated pooled proportions, with corresponding 95% CIs, for retention in care and viral suppression using the DerSimonian and Laird Random Effects (RE) model, with double arcsine transformation to stabilise the variance.15 16 Meta-analyses were performed in MetaXL (V.3.1, EpiGear International, Queensland, Australia) (Barendregt JD, Doi SA. MetaXL User Guide. 2015;Version 3.1). Heterogeneity was assessed with the I2 statistic.17 We planned analyses by relevant subgroups, focusing on age groups and study endpoints, to examine anticipated variation in the outcomes of interest.
Findings
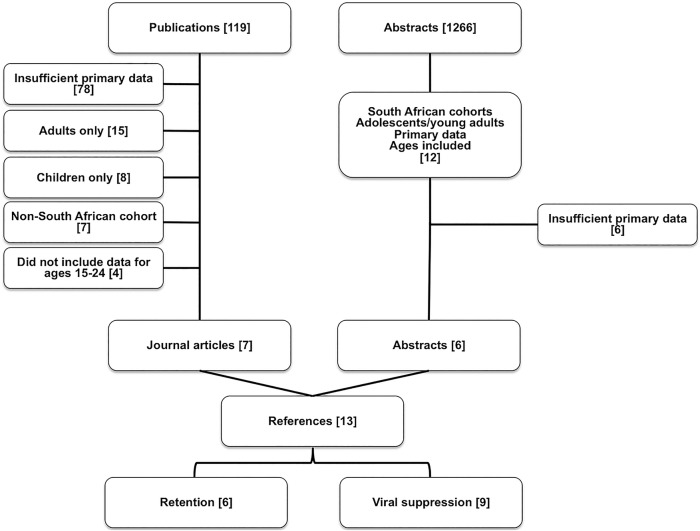
Our systematic review found 119 relevant articles in the published literature addressing the continuum of care for HIV-infected adolescents in South Africa. As indicated in figure 1, 78 were excluded due to insufficient primary data reported; 15 were excluded since they contained only adult data; eight were excluded for only including paediatric data (with ages <15 years); seven were excluded since they reported cohorts outside of South Africa and an additional four were excluded since they did not report results for the ages 15–24 in the primary outcomes. We searched 1266 conference abstracts from IAS/AIDS and CROI from 1 January 2005 to 31 July 2015 and found 12 that included primary data from South African cohorts involving adolescent retention or viral suppression. Six of these studies were excluded for insufficient primary outcome data. Overall, there were 13 sources that contained sufficient data to be included in the meta-analysis with two studies containing both retention and viral suppression data.
Figure 1.
Flow diagram of literature reviewed for meta-analysis of retention in care and viral suppression for HIV-infected adolescents and young adults in South Africa published or presented between 1 January 2005 and 31 July 2015.
Systematic review
Overview of HIV Infections among Adolescents and Young Adults in South Africa.
Currently, HIV-infected adolescents and young adults in South Africa are a combination of two separate epidemics, perinatally HIV-infected youth and newly acquired HIV among adolescents and young adults. The South African National HIV Prevalence, Incidence, and Behavior Survey in 2012 estimated that up to a total of 720 000 youth aged 15–24 are infected with HIV.6 By 2013 statistics, South Africa estimated that 8.5% of the estimated 10 203 329 adolescents and young adults aged 15–24 were infected with HIV totalling 867 283 infections.18 Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated there were 320 000 HIV-infected adolescents aged 10–19 in 2013.19 These estimates contain both perinatally infected and non-perinatally infected youth; however, they do not extrapolate on the percentages in each category.
Perinatal infections
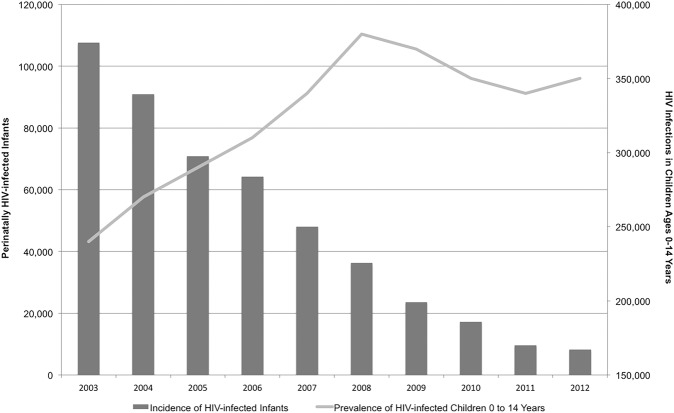
In 2003, before the implementation of the South African National HIV Treatment Programme, national HIV prevalence among women attending antenatal clinics was 27.9%; yet, the number of pregnant women who tested for HIV was <50%.20 21 Low testing rates and limited prevention of mother-to-child HIV transmission (PMTCT) services led to a mother-to-child HIV transmission rate of 23% in 2003.22 With 1 677 415 infants born in South Africa in 2003,21 23 this translates to 107 639 newly HIV-infected infants. By 2005, after ART became available in South Africa, the national HIV antenatal clinic prevalence rate rose to 30.2%, but the mother-to-child transmission rate decreased to 17%. The 1 380 496 births in 2005 translated into 70 875 newly HIV-infected infants. By 2012, the expansion of ART services and PMTCT, including updated PMTCT guidelines in 2008 and 2010, led to decreasing mother-to-child HIV transmission rates (2.4%); however, with 1 168 403 births and a 29.5% antenatal clinic prevalence rate, this translated into 8272 newly HIV-infected infants.6 7 21 24 The decline in infections from 2003 to 2012 represents a 92% reduction in new paediatric HIV infections in 9 years, as indicated in figure 2.6 7 21 Since the rollout of ART in South Africa, there has been a steady decrease in the mother-to-child transmission rate. UNAIDS estimated that in 2014 there were 65 000 averted perinatal HIV infections by PMTCT in South Africa.1
Figure 2.
Systematic review: Perinatally HIV-infected infants in South Africa, total HIV-infected children aged 0–14 and the approaching wave of HIV-infected adolescents expected to enter adult care by 2023.
Incidence of HIV among adolescents
A national survey in 2012 estimated that 139 000 new infections occurred among youth aged 15–24.6 The number of new infections disproportionally affects females who account for 81% of these new infections.6 The overall HIV incidence was 2.5 in females aged 15–24 compared with 0.6 for males of the same age.6
Antiretroviral therapy
In 2012, a nationally representative survey conducted in South Africa estimated 103 000 (95% CI 72 000 to 144 000) youth aged 15–24 were on ART, comprising 14% of HIV-infected adolescents in South Africa and 1.5% of South Africans on ART.6 Estimates in large urban academic centres in Johannesburg, Cape Town and Durban have differing percentages of their total ART programme that are comprised of adolescents: Johannesburg at 6.7% for those aged 15–24; Cape Town at 0.8% for those 12–17 and Durban 11.1% for those aged 10–19 (M Archary. KwaZulu-Natal Adolescent ART data. Personal Communication 2015; D Evans. Themba Lethu Clinic Adolescent Data. Personal Communication 2015; C Orrell. Masi and Gugulethu Adolescent Data. Personal Communication 2015). Based on 2013 population estimates, we estimate that 124 070 (14%; 95% CI 10% to 20%) adolescents and young adults aged 15–24 in South Africa are receiving ART (figure 4).
Figure 4.
Estimate of the absolute number of South African HIV-infected adolescents and young adults aged 15–24 completing the HIV continuum of care.
Meta-analysis
Retention in care
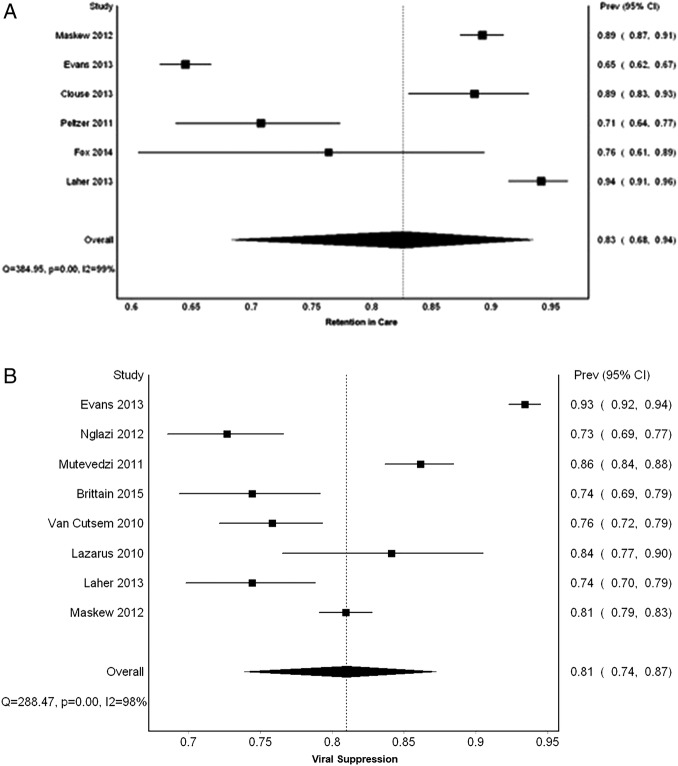
Results of our meta-analysis of six studies estimate that the proportion of South African HIV-infected adolescents on ART who were retained in care within the first 1– 2 years of therapy is 83% (95% CI 68% to 94%; I2=99%), as indicated in table 1 and figures 3A and 4.25–30 No differences were seen when comparing this proportion by timing end point or age group.
Table 1.
Published literature from 1 January 2005 to 31 August 2015, addressing the continuum of care for HIV-infected adolescents and young adults in South Africa
| HIV infections | |||||
| Age | HIV infected | Per cent | |||
| Shisana, 20146 31 | 15–24 | 720 000 | 7.1* | ||
| Statistics South Africa, 201318 | 15–24 | 867 283 | 8.5 | ||
| UNAIDS, 201318 19 | 10–19 | 320 000 | 3.1† | ||
| Antiretroviral therapy | |||||
| Age | HIV infected | On ART | Per cent | ||
| Shisana, 20146 | 15–24 | 720 000 | 103 000 | 14 | |
| Retention in care | |||||
| Age | N | Retained | Per cent | End point | |
| Maskew, 201229 | 18–29 | 1140 | 1018 | 89 | Retained 24 months |
| Evans, 201326 | 15–24 | 1941 | 1252 | 65 | Retained 12 months |
| Clouse, 201325 | 18–29 | 158 | 140 | 89 | Retained 12 months |
| Peltzer, 201130 | 18–28 | 171 | 121 | 71 | Retained 12 months |
| Fox, 201432 | 18–29 | 34 | 26 | 76 | Cross-sectional |
| Laher, 201328 | 9–26 | 360 | 339 | 94 | Cross-sectional |
| Boyles, 201133 | Adults | 1803 | 82 | Cross-sectional | |
| Van Cutsem, 201034 | Adults | 6411 | 92 | Cross-sectional | |
| Meta-analysis of retention | N | Retained | Per cent | ||
| 3604 | 2896 | 83% (95%CI 68% to 93%);I2=99% | |||
| Viral suppression | |||||
| Age | N | Suppressed | Per cent | End point‡ | |
| Evans, 201326 | 15–24 | 1941 | 1813 | 93 | 6 months |
| Nglazi, 201235 | 9–28 | 472 | 343 | 73 | 6 months |
| Mutevedzi, 201136 | 16–24 | 808 | 696 | 86 | 12 months |
| Brittain, 201537 | 9–14 | 305 | 227 | 75 | Cross-sectional |
| Van Cutsem, 201038 | 10–24 | 546 | 414 | 76 | 12 months |
| Lazarus, 201039 | 11–19 | 107 | 90 | 84 | Cross-sectional |
| Maskew, 201229 | 18–29 | 1738 | 1407 | 81 | 6 months |
| Laher, 201328 | 9–26 | 360 | 268 | 74 | Cross-sectional |
| Davies, 2014*40 | 10–13 | 2161 | 1664 | 77 | Cross-sectional |
| Meta-analysis of viral suppression | N | Retained | Per cent | ||
| 6227 | 5258 | 81% (95%CI 74% to 87%); I2=98% | |||
Bolded citations were included in meta-analysis.
*Calculated based on population data from 2012.
†Calculated based on population data from 2013.
‡Viral Suppression as Viral Load <400 copies/mL.
Figure 3.
(A) Meta-analysis retention: Forest plot of the proportion of HIV-infected adolescents and young adults in South Africa retained in care. (B) Meta-analysis viral suppression: Forest plot of the proportion of virally suppressed HIV-infected adolescents and young adults in South Africa.
Viral suppression
Based on our meta-analysis of eight studies, we estimate the proportion of South African adolescents and young adults on ART who were virally suppressed to be 81% (95% CI 74% to 87%; I2=98%), as indicated by table 1 and figures 3B and 4.26 28 29 36–39 41 No differences were seen when comparing this proportion by timing end point or age group.
Mortality
Globally, from 2005 to 2012, the overall number of HIV-related deaths decreased by 30%; however, deaths in HIV-infected adolescents over the same time period increased by 50%.42 In 2013, in South Africa alone, there were more than 9500 deaths among HIV-infected adolescents.19 Despite having low ART initiation rates, lower retention in care and more virological failure, multiple cohorts in South Africa did not see an increase in mortality rates among adolescents and young adults in care and on ART.25 26 30 36 43–48
Discussion
South Africa has had a dramatic increase in the number of HIV-infected individuals on ART in the last several years; however, the percentage of adolescents fully benefiting from treatment remains low. Compared with HIV-infected adolescents in the USA, South Africa has a lower percentage of youth receiving ART treatment (14% vs 25%), yet it does have similar rates of retention in care (11% vs 11%) and higher rates of viral suppression (10% vs 6%).13 49 South Africa also has a higher number of infections at 867 283 compared with 78 949 in the USA. Despite a higher viral suppression rate, 709 773 more adolescents are not virally suppressed in South Africa compared with the USA. Descriptions of the continuum of care for HIV-infected adolescents in others settings have not been described, limiting further comparisons.
South African studies with direct comparison between adolescents and adults indicate lower retention and viral suppression among youth. Six studies in South Africa documented significantly poorer retention in care rates for adolescents compared with adults with ORs ranging from 1.55 to 2.25.25 26 33 34 38 50 For example, a large cohort from Gauteng and Mpumalanga with more than 42 000 patients showed retention rates for adolescents aged 15–24 at 65% compared with adults over 25 years of age at 78%.26 Five separate South African observational cohorts reported significantly lower HIV viral suppression rates among adolescents and young adults (range: 73–93%) after ART initiation compared with adults (range: 88 –97%).26 36 38 50 51
Despite HIV-infected adolescents having lower rates of retention in care and viral suppression rates compared with adults, mortality rates among adolescents in comparative studies are similar to adults.25 26 30 36 43–48 This likely reflects the delayed mortality in newly HIV-infected youth that die after ageing into adulthood. With high retention and viral suppression rates among adolescents in South Africa, strategies to improve HIV diagnosis and early ART initiation in adolescents could drastically improve the continuum of care for youth in South Africa.
Low ART treatment rates among adolescents currently are inversely related to the high number of new HIV infections in this age group. Youth who are newly infected are less likely to qualify for ART based on National HIV Treatment Guidelines (CD4 below 500 cells/mm3 at the time of this writing) due to asymptomatic disease and higher CD4 counts.52 The cohort of sexually active, HIV-infected and viremic youth could have a large impact on HIV transmission rates in South Africa and warrants consideration for public health investment.
Expansion of proven treatment and prevention programmes for adolescents and young adults could reduce the high incidence of HIV infections in this age group. Recent evidence has shown the benefit of test-and-treat strategies for prevention of HIV regardless of clinical stage or absolute CD4, assuming individuals stay in care.53 Clinical evidence is also building for initiation of ART at higher CD4 counts.54 55 Recently, the WHO recommended universal treatment for HIV-infected individuals, including adolescents and young adults.52 56 57 In addition, pre-exposure prophylaxis, recently endorsed by the WHO, has been shown to decrease HIV transmission in several cohorts and may be useful in people engaging in high-risk sexual behaviour.57–60 However, concern about poor adherence among adolescents suggests that this strategy will require careful planning, monitoring and research.
The high HIV incidence rate among South African females aged 15–24 in particular calls for HIV prevention efforts in this group. Incidence rates for all other age groups have decreased over the past decade; however, incidence remains steadily high in females aged 15–24.2 6 The risk of HIV acquisition among adolescent females is thought to be higher if there is a greater than 5-year difference in the age between female and male sexual partners.6 61 Several studies suggest that this age discrepancy is driven by sexual relationships between older males and younger females as transactional sex.62–70 However, a recent study from rural KwaZulu-Natal, South Africa did not see an increase in HIV incidence among younger females with older male partners.71
There is an expected oncoming wave of perinatally HIV-infected children ageing into adolescence who will require adult services in the next 5–10 years. Infants surviving and initiating ART in the mid-2000s are now transitioning into adolescence. Additionally, ∼25–30% of perinatally infected children are slow progressors who can survive undiagnosed and untreated well into adolescence.72 73 As this wave of perinatally HIV-infected adolescents mature, combined with the high number of new infections among older adolescents, a large number of adolescents will transfer from paediatric or adolescent-based clinics to adult services.74–77 These already overwhelmed institutions are poorly prepared for the increased patient population. In addition, these individuals have different needs from patients currently in care at adult services. With many chronic illnesses, this transition is often associated with lapses in adherence and poor clinical outcomes.78–81 Revolving care providers, lack of youth-friendly services, rigid scheduling and decreased adult caregiver involvement have marred this transition process.82–84 More research is needed to investigate optimal practices for transitioning HIV-infected youth to adult services.
This review has several limitations. First, it relies on published data and therefore could be subject to publication bias, likely biasing adolescents to lower rates at each step of the continuum of care. We did, however, obtain estimates of the continuum of care from primary data from several large urban settings in Durban, Johannesburg, Pietermaritzburg and Cape Town. In addition, this review relied on retrospective observational data, which were mostly from large urban centres. It is possible that this data is not representative and generalisable to adolescents in rural settings. The analysis for viral suppression assumes that all participants in each cohort are alive, in care and do not have missing data. Since this was a retrospective review of the literature, we were unable to include clinical data such as CD4 improvement, weight gain and documentation of opportunistic infections. There was also considerable heterogeneity in the estimates for retention in care and viral suppression, which could not be explained by the variation in study endpoints or age group. Additional efforts are needed to try and explore the sources of this heterogeneity.
Conclusion
The oncoming wave of HIV-infected adolescents entering adult care could put a strain on already limited resources. These adolescents have different needs than adults and have lower viral suppression and retention rates. More research must be conducted to optimise retention in care, ART treatment and transition care for HIV-infected adolescents in South Africa.
Acknowledgments
The authors would like to thank Thobekile Sibaya, Denise Evans and Catherine Orrell for assisting with data collection. They would also like to thank Holly Zanoni for her assistance with the figures and formatting.
Footnotes
Handling editor: Seye Abimbola
Contributors: BCZ conceptualised and designed the study, performed the literature review, assisted with the analysis, drafted the initial manuscript, reviewed and revised the manuscript and approved the final manuscript as submitted. SB and JEH carried out the initial analyses, reviewed and revised the manuscript and approved the final manuscript as submitted. MA and ITK provided information on data sources, method design and critically reviewed the manuscript and approved the final manuscript as submitted.
Funding: BCZ is supported by the National Institute of Health (grant number 5T32AI 052074).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS Country Report: South Africa 2015. http://www.unaids.org/en/regionscountries/countries/southafrica [PubMed]
- 2.Joint United Nations Programme on HIV/AIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013 Joint United Nations Programme on HIV/AIDS, 2013. http://www.unaids.org [PubMed] [Google Scholar]
- 3.Chigwedere P, Seage GR III, Gruskin S et al. . Estimating the lost benefits of antiretroviral drug use in South Africa. J Acquir Immune Defic Syndr 2008;49:410–15. 10.1097/QAI.0b013e31818a6cd5 [DOI] [PubMed] [Google Scholar]
- 4.Ojikutu B, Makadzange AT, Gaolathe T. Scaling up ART treatment capacity: lessons learned from South Africa, Zimbabwe, and Botswana. Curr Infect Dis Rep 2008;10:69–73. 10.1007/s11908-008-0012-0 [DOI] [PubMed] [Google Scholar]
- 5.Newell ML, Coovadia H, Cortina-Borja M et al. . Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004;364:1236–43. 10.1016/S0140-6736(04)17140-7 [DOI] [PubMed] [Google Scholar]
- 6.Shisana O, Rehle T, Simbayi LC et al. . South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town, HSRC Press, 2014, pp 57–60, 66–68. [Google Scholar]
- 7.Goga AE, Dinh TH, Jackson DJ, for the SAPMTCTE study group. Evaluation of the Effectiveness of the National Prevention of Mother-to-Child Transmission (PMTCT) Programme Measured at Six Weeks Postpartum in South Africa, 2010. South African Medical Research Council, National Department of Health of South Africa and PEPFAR/US Centers for Disease Control and Prevention, 2012:2. [Google Scholar]
- 8.South African National Department of Health. National Consolidated Guidelines for the Prevention of Mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents, and adults. Pretoria, South Africa: Department of Health, 2014. [Google Scholar]
- 9.Mossong J, Grapsa E, Tanser F et al. . Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS 2013;27: 2471–9. 10.1097/01.aids.0000432475.14992.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaba B, Marston M, Crampin AC et al. . Age-specific mortality patterns in HIV-infected individuals: a comparative analysis of African community study data. AIDS 2007;21(Suppl 6):S87–96. 10.1097/01.aids.0000299415.67646.26 [DOI] [PubMed] [Google Scholar]
- 11.Zaidi J, Grapsa E, Tanser F et al. . Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS 2013;27:2301–5. 10.1097/QAD.0b013e328362e832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilmarx PH, Mutasa-Apollo T. Patching a leaky pipe: the cascade of HIV care. Curr Opin HIV AIDS 2013;8:59–64. [DOI] [PubMed] [Google Scholar]
- 13.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014;28:128–35. 10.1089/apc.2013.0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner EM, McLees MP, Steiner JF et al. . The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800. 10.1093/cid/ciq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barendregt JJ, Doi SA, Lee YY et al. . Meta-analysis of prevalence. J Epidemiol Community Health 2013;67:974–8. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics South Africa. Mid year population estimates: 2013. Pretoria, South Africa: Statistics South Africa; 2013. http://www.statssa.gov.za/publications/SAStatistics [Google Scholar]
- 19.The Joint United Nations Programme on HIV/AIDS (UNAIDS) HIV Estimates, 2013. http://allintoendadolescentaids.org/wp-content/uploads/2015/02/South-Africa.pdf. [PubMed]
- 20.Joint United Nations Programme on HIV/AIDS. Republic of South Africa Country Progress Report on the Declaration of Commitment on HIV/AIDS 2010 Report South African National Department of Health Health, 2010. [Google Scholar]
- 21.South African National Department of Health. The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey. Pretoria, South Africa: South African National Department of Health, 2012. [Google Scholar]
- 22.Sherman GG, Lilian RR, Bhardwaj S et al. . Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. S Afr Med J 2014;104(Suppl 1):235–8. 10.7196/SAMJ.7598 [DOI] [PubMed] [Google Scholar]
- 23.Statistics South Africa. Recorded live births: 2013. Pretoria, South Africa: Statistics South Africa, 2013. http://www.statssa.gov.za/publications/SAStatistics [Google Scholar]
- 24.Statistics South Africa (Stats SA). Recorded Live Births 2013. Statistical Release P0305. Page 9 http://www.statssa.gov.za/publications/P0305/P03052013.pdf [Google Scholar]
- 25.Clouse K, Pettifor AE, Maskew M et al. . Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2013;62:e39–46. 10.1097/QAI.0b013e318273ac48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans D, Menezes C, Mahomed K et al. . Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses 2013;29:892–900. 10.1089/aid.2012.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MP, Shearer K, Maskew M et al. . Treatment outcomes after 7 years of public-sector HIV treatment. AIDS 2012;26:1823–8. 10.1097/QAD.0b013e328357058a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laher F, Hornschuh S, Otwombe K et al. . Outcomes of adolescents and young adults receiving antiretroviral therapy in Soweto, South Africa. Paper presented at: 7th IAS Conference on HIV Pathogenesis and Treatment Abstract no. MOPE0562013; Kuala Lumpur, Malaysia. [Google Scholar]
- 29.Maskew M, Brennan AT, MacPhail AP et al. . Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care (Chic) 2012;11:57–65. 10.1177/1545109711421641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peltzer K, Ramlagan S, Khan MS et al. . The social and clinical characteristics of patients on antiretroviral therapy who are ‘lost to follow-up’ in KwaZulu-Natal, South Africa: a prospective study. SAHARA J 2011;8:179–86. 10.1080/17290376.2011.9725002 [DOI] [PubMed] [Google Scholar]
- 31.Statistics South Africa. South African Statistics 2012. Pretoria, South Africa: Statistics South Africa, 2012. http://www.statssa.gov.za/publications/SAStatistics [Google Scholar]
- 32.Fox MP, Shearer K, Maskew M et al. . Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS ONE 2014;9:e110252 10.1371/journal.pone.0110252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyles TH, Wilkinson LS, Leisegang R et al. . Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS ONE 2011;6:e19201 10.1371/journal.pone.0019201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Cutsem G, Ford N, Hildebrand K et al. . Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS ONE 2011;6:e14684 10.1371/journal.pone.0014684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nglazi MD, Kranzer K, Holele P et al. . Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis 2012;12:21 10.1186/1471-2334-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutevedzi PC, Lessells RJ, Rodger AJ et al. . Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS ONE 2011;6:e21795 10.1371/journal.pone.0021795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brittain KA-A, N.A, Hoare J, Bekker, L-G, Nuttall J., Roux P, Stein D.J, Zar H.J, Myer L Antiretroviral therapy adherence in perinatally-infected adolescents in Cape Town, South Africa. Paper presented at: International AIDS Society (IAS) Abstract no. MOPEB2032015; Vancouver, CA. [Google Scholar]
- 38.Van Cutsem G KL, Abrahams M, Kerschberger B et al. . Outcomes in children, adolescent, youth and adults on ART in Khayelitsha. Paper presented at: AIDS 2010—XVIII International AIDS Conference Abstract no. THPE0170; Vienna, 2010. [Google Scholar]
- 39.Lazarus EM OK, Mohapi L, Cescon A et al. . Effect of baseline immunological condition, virological response and duration of HAART on growth in HIV-infected adolescents. Paper presented at: XVIII International AIDS Conference: Abstract no. MOAB04032010; Vienna. [Google Scholar]
- 40.Davies M-A SK, Technau K, Phiri S et al. . Outcomes of Perinatally HIV-infected Adolescents on Antiretroviral Therapy in Southern Africa. Paper presented at: Conference on Retrovirology and Opportunistic Infections (CROI) Abstract no: 9302014; Boston, MA. [Google Scholar]
- 41.Davies MA, May M, Bolton-Moore C et al. . Prognosis of children with HIV-1 infection starting antiretroviral therapy in Southern Africa: a collaborative analysis of treatment programs. Pediatr Infect Dis J 2014;33:608–16. 10.1097/INF.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Orginization. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 43.Ahonkhai AA, Noubary F, Munro A et al. . Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS ONE 2012;7:e32993 10.1371/journal.pone.0032993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies MA, Keiser O, Technau K et al. . Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J 2009;99:730–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Fatti G, Meintjes G, Shea J et al. . Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr 2012;61:e50–8. 10.1097/QAI.0b013e31826a6aee [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann CJ, Fielding KL, Johnston V et al. . Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr 2011;58:269–76. 10.1097/QAI.0b013e31823219d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacPherson P, Moshabela M, Martinson N et al. . Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg 2009;103:588–93. 10.1016/j.trstmh.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 48.Vella V, Govender T, Dlamini S et al. . Retrospective study on the critical factors for retaining patients on antiretroviral therapy in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 2010;55:109–16. 10.1097/QAI.0b013e3181e7744e [DOI] [PubMed] [Google Scholar]
- 49.Kapogiannis B XJ, Mayer K, Loeb J et al. . and the ATN/SMILE Collaborative. The HIV continuum of care for adolescents and young adults (12–24 years) attending 13 urban Us centers of the NICHD-ATN-CDC-HRSA SMILE collaborative. Paper presented at: 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention2015; Vancover. [Google Scholar]
- 50.Nglazi MD, Lawn SD, Kaplan R et al. . Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr 2011;56:e1–8. 10.1097/QAI.0b013e3181ff0bdc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barth RE, van der Meer JT, Hoepelman AI et al. . Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis 2008;27:977–84. 10.1007/s10096-008-0534-2 [DOI] [PubMed] [Google Scholar]
- 52.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. In: Services. Department of Health and Human Services; http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdoles2015 [Google Scholar]
- 53.Cohen MS, Chen YQ, McCauley M et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lundgren JD, Babiker AG, Gordin F et al. , INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temprano ANS, Study G, Danel C et al. . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–22. 10.1056/NEJMoa1507198 [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 57.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 58.Molina JCC, Spire B, vPialoux G et al. . On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial: Abstract 23LB. Conference on Retrovirology and Opportunistic Infections (CROI); Seattle, WA, 2015. [Google Scholar]
- 59.Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–99. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baeten JM, Donnell D, Ndase P et al. . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ott MQ, Barnighausen T, Tanser F et al. . Age-gaps in sexual partnerships: seeing beyond ‘sugar daddies’. AIDS 2011;25:861–3. 10.1097/QAD.0b013e32834344c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdool Karim Q, Abdool Karim SS, Singh B et al. . Seroprevalence of HIV infection in rural South Africa. AIDS 1992;6:1535–9. 10.1097/00002030-199212000-00018 [DOI] [PubMed] [Google Scholar]
- 63.Anderson RM, May RM, Ng TW et al. . Age-dependent choice of sexual partners and the transmission dynamics of HIV in Sub-Saharan Africa. Philos Trans R Soc Lond B Biol Sci 1992;336:135–55. 10.1098/rstb.1992.0052 [DOI] [PubMed] [Google Scholar]
- 64.Dunkle KL, Jewkes RK, Brown HC et al. . Transactional sex among women in Soweto, South Africa: prevalence, risk factors and association with HIV infection. Soc Sci Med 2004;59:1581–92. 10.1016/j.socscimed.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 65.Wojcicki JM. “She drank his money”: survival sex and the problem of violence in taverns in Gauteng province, South Africa. Med Anthropol Q 2002;16:267–93. 10.1525/maq.2002.16.3.267 [DOI] [PubMed] [Google Scholar]
- 66.Cluver L, Orkin M, Boyes M et al. . Transactional sex amongst AIDS-orphaned and AIDS-affected adolescents predicted by abuse and extreme poverty. J Acquir Immune Defic Syndr 2011;58:336–43. 10.1097/QAI.0b013e31822f0d82 [DOI] [PubMed] [Google Scholar]
- 67.Oldenburg CE, Perez-Brumer AG, Reisner SL et al. . Global burden of HIV among men who engage in transactional sex: a systematic review and meta-analysis. PLoS ONE 2014;9:e103549 10.1371/journal.pone.0103549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Heijden I, Swartz S. ‘Something for something’: the importance of talking about transactional sex with youth in South Africa using a resilience-based approach. Afr J AIDS Res 2014;13:53–63. 10.2989/16085906.2014.886602 [DOI] [PubMed] [Google Scholar]
- 69.Watt MH, Aunon FM, Skinner D et al. . “Because he has bought for her, he wants to sleep with her”: alcohol as a currency for sexual exchange in South African drinking venues. Soc Sci Med 2012;74:1005–12. 10.1016/j.socscimed.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zembe YZ, Townsend L, Thorson A et al. . “Money talks, bullshit walks” interrogating notions of consumption and survival sex among young women engaging in transactional sex in post-apartheid South Africa: a qualitative enquiry. Global Health 2013;9:28 10.1186/1744-8603-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harling G, Newell ML, Tanser F et al. . Age-Disparate Relationships and HIV Incidence Amongst Rural South Africa Women. Paper presented at: Conference on Retrovirology and Opportunistic Infections (CROI) Abstract No. 1452014; Boston, MA. [Google Scholar]
- 72.Ferrand RA, Corbett EL, Wood R et al. . AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS 2009;23:2039–46. 10.1097/QAD.0b013e32833016ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marston M, Becquet R, Zaba B et al. . Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol 2011;40:385–96. 10.1093/ije/dyq255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikard K, Janney J, Hsu LC et al. . Estimation of unmet need for HIV primary medical care: a framework and three case studies. AIDS Educ Prev 2005;17(Suppl B):26–38. 10.1521/aeap.2005.17.SupplementB.26 [DOI] [PubMed] [Google Scholar]
- 75.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008;372:293–9. 10.1016/S0140-6736(08)61113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Committee On Pediatric Aids. Transitioning HIV-infected youth into adult health care. Pediatrics 2013;132:192–7. 10.1542/peds.2013-1073 [DOI] [PubMed] [Google Scholar]
- 77.Andiman WA. Transition from pediatric to adult healthcare services for young adults with chronic illnesses: the special case of human immunodeficiency virus infection. J Pediatr 2011;159:714–19. 10.1016/j.jpeds.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 78.Brousseau DC, Owens PL, Mosso AL et al. . Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010;303:1288–94. 10.1001/jama.2010.378 [DOI] [PubMed] [Google Scholar]
- 79.Cervia JS. Easing the transition of HIV-infected adolescents to adult care. AIDS Patient Care STDS 2013;27:692–6. 10.1089/apc.2013.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiener LS, Kohrt BA, Battles HB et al. . The HIV experience: youth identified barriers for transitioning from pediatric to adult care. J Pediatr Psychol 2011;36:141–54. 10.1093/jpepsy/jsp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fish R, Judd A, Jungmann E et al. . Mortality in perinatally HIV-infected young people in England following transition to adult care: an HIV Young Persons Network (HYPNet) audit. HIV Med 2014;15:239–44. 10.1111/hiv.12091 [DOI] [PubMed] [Google Scholar]
- 82.Hunt SE, Sharma N. Transition from pediatric to adult care for patients with sickle cell disease. JAMA 2010;304:408–9; author reply 409 10.1001/jama.2010.1026 [DOI] [PubMed] [Google Scholar]
- 83.Lowenthal ED, Marukutira T, Tshume O et al. . Parental absence from clinic predicts human immunodeficiency virus treatment failure in adolescents. JAMA Pediatr 2015;169:498–500. 10.1001/jamapediatrics.2014.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams PL, Storm D, Montepiedra G et al. . Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics 2006;118:e1745–57. 10.1542/peds.2006-0493 [DOI] [PubMed] [Google Scholar]