Fig 5. Vpu is inserted into the bacterial inner membrane with its C-terminal domain within the cytoplasm.
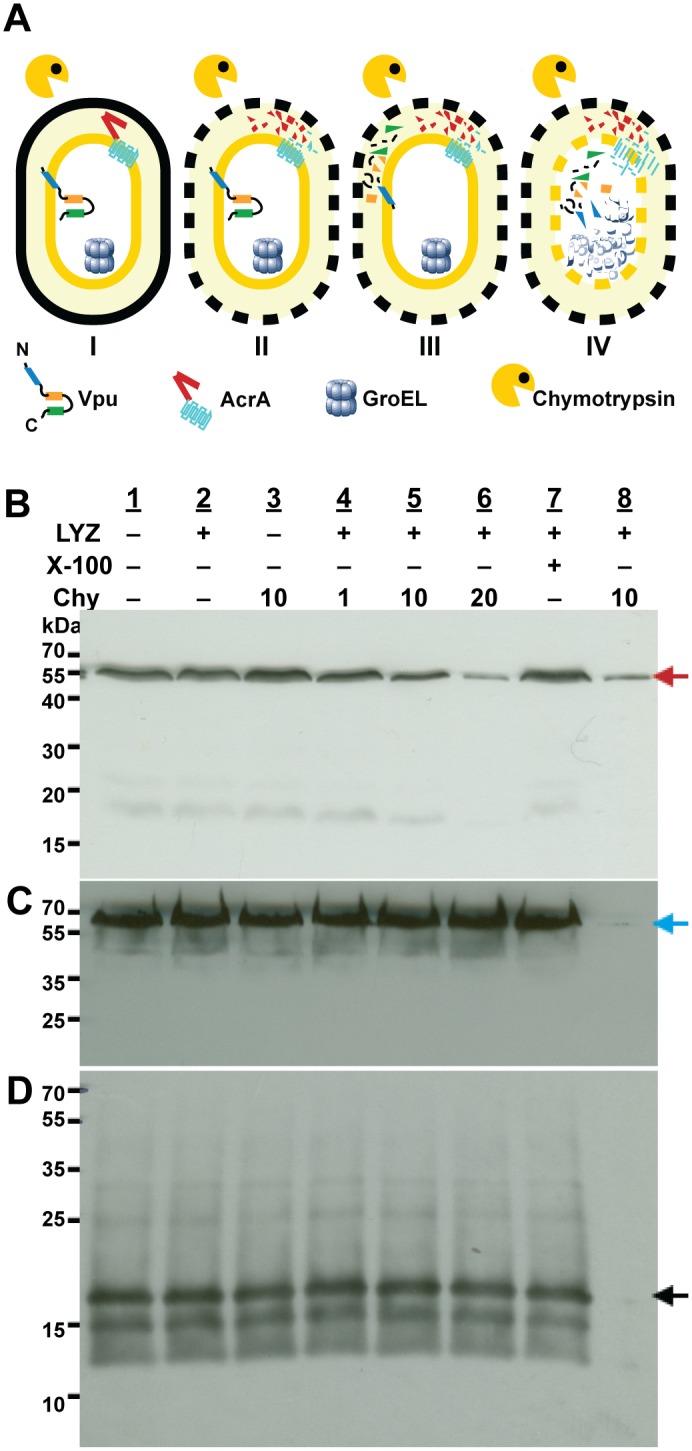
Panel A depicts the possible outcomes when E. coli cells (I) and protoplasts (II III and IV) that express Vpu are digested with chymotrypsin alone (I-III) and in the the presence of Triton X-100 (IV). If the C-terminal domain of Vpu is protected by the inner membrane (I and II), it is expected to be resistant to chymotrypsin digestion, similar to the case of the cytoplasmic resident protein GroEL. If the C-terminal domain of Vpu is exposed at the periplasmic space of the cells (III), it is expected to be digested in the same way as AcrA. All three proteins should undergo chymotrypsin cleavage when the inner membranes are permeabilized by the addition of Triton X-100 (IV). E. coli cells (lanes 1 and 3) and protoplasts (lanes 2 and 4–8) were incubated without chymotrypsin (lanes 1, 2, and 7) or with the indicated concentrations of chymotrypsin (μg/mL); in the presence of 1% Triton X-100 (lanes 7 and 8) or its absence (lanes 1–6). Following treatments, cells were solubilized in the presence of SDS sample buffer and protein samples were resolved by SDS-PAGE followed by immunoblotting with antibodies against the periplasmic domain of the inner-membrane protein AcrA (Panel B, red arrow), cytoplasmic protein GroEL (Panel C, blue arrow) or Vpu C-terminal domain (Panel D, black arrow).
