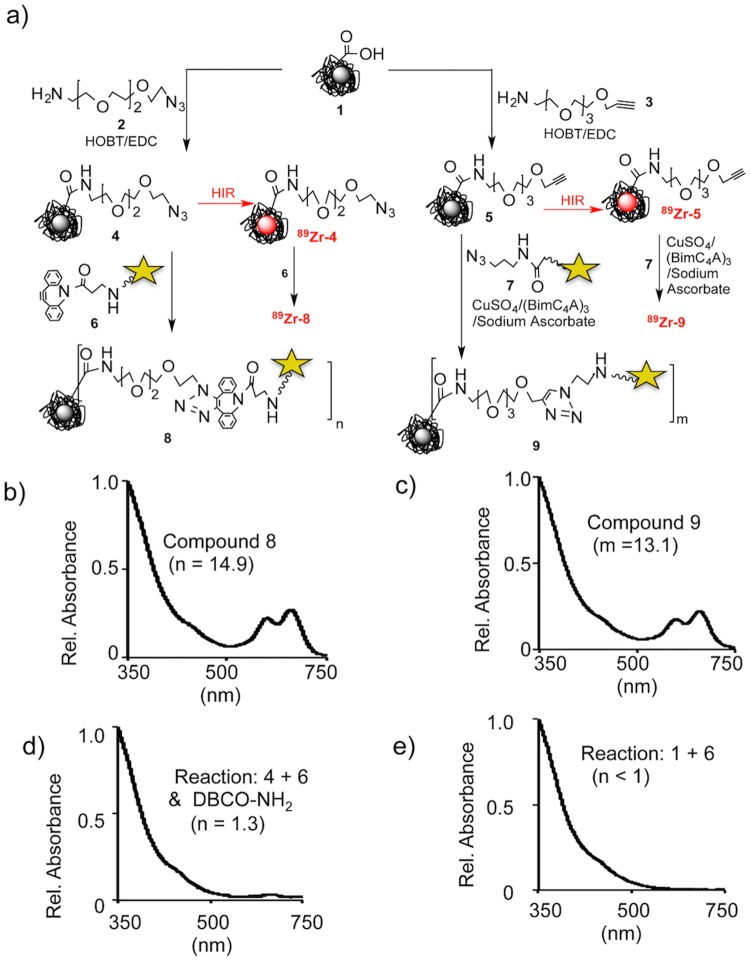
Fig 2. Surface functionalization of Feraheme (FH, 1) with azide or alkyne groups and radiolabeling functionalized NPs by HIR.
a) Using FH (1), the Azide-FH (4) and Alkyne-FH (5) were synthesized. Portions of Azide-FH (4) and Alkyne-FH (5) were then radiolabeled by HIR, yielding 89Zr-Azide-FH (89Zr-4) and 89Zr-Alkyne-FH (89Zr-5). To determine reactive azide or reactive alkynes, NPs were reacted with the appropriate click reactive Cy5.5 fluorochromes, with Cy5.5s shown as the yellow stars of Fig 1. After removal of the unreacted Cy5.5s (DBCO-Cy5.5, 6 or Azide-Cy5.5, 7), the number of Cy5.5’s per NP was determined from absorption spectra examples of which are shown in Fig 2b–2e. Controls for covalent binding were a reaction of FH (1) and DBCO-Cy5.5 (6) and a reaction of Azide-FH (4) and DBCO-Cy5.5 (6) preoccupied with DBCO-NH2. Values in parentheses are the numbers of reactive groups per NP with values summarized in Table 1.