Abstract
Extracellular adenosine 5’-triphosphate (ATP) performs multiple functions including activation and induction of apoptosis of many cell types. The ATP-hydrolyzing ectoenzyme ecto-nucleotide pyrophosphatase/phosphodiesterase 3 (E-NPP3) regulates ATP-dependent chronic allergic responses by mast cells and basophils. However, E-NPP3 is also highly expressed on epithelial cells of the small intestine. In this study, we showed that E-NPP3 controls plasmacytoid dendritic cell (pDC) numbers in the intestine through regulation of intestinal extracellular ATP. In Enpp3-/- mice, ATP concentrations were increased in the intestinal lumen. pDC numbers were remarkably decreased in the small intestinal lamina propria and Peyer’s patches. Intestinal pDCs of Enpp3-/- mice showed enhanced cell death as characterized by increases in annexin V binding and expression of cleaved caspase-3. pDCs were highly sensitive to ATP-induced cell death compared with conventional DCs. ATP-induced cell death was abrogated in P2rx7-/- pDCs. Accordingly, the number of intestinal pDCs was restored in Enpp3-/- P2rx7-/- mice. These findings demonstrate that E-NPP3 regulates ATP concentration and thereby prevents the decrease of pDCs in the small intestine.
Introduction
Extracellular ATP released by damaged/dying or activated cells regulates several immune responses via purinergic receptors such as P2X and P2Y [1–3]. ATP activates many types of immune cells, including dendritic cells (DCs), macrophages, T cells, B cells, mast cells, and basophils, via P2Y and P2X receptors [4–10]. Furthermore, several lines of evidence have indicated that extracellular ATP induces inflammatory responses in several tissues [11–14]. In addition, ATP induces apoptosis of several cell types [15–21]. Thus, regulation of the extracellular ATP concentration is required to maintain host homeostasis.
Nucleotide converting ectoenzymes, such as ecto-nucleoside triphosphate diphosphohydrolase (E-NTPD) and ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) families, play critical roles in controlling the concentration of extracellular ATP through ATP hydrolysis [22–27]. Additionally, several studies have indicated that nucleotide converting ectoenzymes modulate immune responses. E-NTPD1 and ecto-5'-nucleotidase (CD73) are highly expressed on CD4+ Foxp3+ regulatory T (Treg) cells, and cooperation of E-NTPD1 and CD73 enhances the immunosuppressive activity of Treg cells via the generation of adenosine from extracellular ATP [28,29]. Moreover, E-NTPD1 and CD73 assist B cells to enter the class switch recombination process by converting ATP to adenosine [30]. E-NTPD7 is specifically expressed in epithelial cells of the small intestine, and inhibits ATP-mediated Th17 cell responses [31]. In addition to E-NTPD1, CD73, and E-NTPD7, E-NPP3 was recently shown to modulate immune responses. E-NPP3, which is expressed on activated basophils and mast cells, suppresses ATP-induced activation of basophils and mast cells, and prevents chronic allergic inflammation [10]. Thus, the functions of E-NPP3 have been well established in basophils and mast cells. However, it remains unclear which functions are performed by E-NPP3 expressed on other cell-types.
Plasmacytoid dendritic cells (pDCs), which express CD11c at a low level but highly express PDCA-1 and B220, play an essential role in the host defense against viral infection by secreting large amounts of type I interferon (IFN) and IL-12 [32,33]. pDCs are largely distributed to secondary lymphoid tissues such as lymph nodes and the spleen. However, they are also present in lamina propria of the small intestine, where they express the gut homing receptor C-C chemokine receptor (CCR) 9 [34]. CCR9 expression correlates with the tolerogenic property of pDCs [35]. pDCs in gut-associated lymphoid tissues, such as Peyer’s patch (PP) and mesenteric lymph nodes (MLNs), possess unique properties. These properties include a low level of type I IFN production, suppression of intestinal inflammation and induction of IgA class switching of B cells [36–38]. However, the regulation of intestinal pDC activity remains unclear.
In this study, we investigated the immunomodulatory functions of E-NPP3 in the intestine where E-NPP3 was highly expressed. Mice lacking E-NPP3 showed an elevated concentration of luminal ATP in the small intestine and a decrease in pDC numbers specifically in PPs and lamina propria of the small intestine. pDCs were highly sensitive to ATP-induced cell death that was dependent on P2X7. Accordingly, introduction of P2rx7 deficiency in Enpp3-/- mice restored the pDC number in the intestine. These findings demonstrate that E-NPP3, which is highly expressed in epithelial cells of the small intestine, plays a critical role in the maintenance of pDC cell numbers through hydrolysis of luminal ATP.
Materials and methods
Mice
The protocols used for all animal experiments in this study were approved by the Animal Research Committee of Osaka University, Japan (No. 23-076-01). Enpp3-/- and P2rx7-/- mice were generated as described previously [10,13]. KitW-sh/W-sh mice were kindly provided by Dr. H. Suto (Atopy Research Center, Juntendo University, Japan). These mice were backcrossed to BALB/c for at least seven generations. BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Mutant mice and their wild-type littermates at 8–12 weeks of age were used in experiments. These mice were maintained under specific pathogen-free conditions. For some experiments, germ-free BALB/c mice were purchased from Clea (Tokyo, Japan).
Reagents
An annexin V staining kit, active caspase-3 staining kit, anti-mouse PerCP/Cy5.5-CD45RA (14.8) and CD16/32 (2.4.G2) antiboies were purchased from BD Biosciences (San Diego, CA, USA). Anti-mouse PE-CD45 (30-F11), Pacific Blue-CD45 (30-F11), PerCP/Cy5.5-CD4 (GK1.5), FITC-CD4 (GK1.5), FITC-CD8a (53–6.7), APC/Cy7-CD45R/B220 (RA3-6B2), FITC-CD45R/B220 (RA3-6B2), Alexa Fluor 647-CD317 (129C1), PE/Cy7-CD11c (N418), FITC-CD11c (N418), APC-FcεRI (MAR-1), PE/Cy7-CD117 (2B8) and PE-Siglec H (551) antibodies were purchased from Biolegend (San Diego, CA, USA). Anti-mouse FITC-CCR9 (eBioCw-1.2) antibody was purchased from eBioscience (San Diego, CA, USA). Anti-mouse FITC-CD3e (145-2C11) antibody was purchased from Tonbo biosciences (San Diego, CA, USA).
Quantitative RT- PCR
Total RNA was isolated using TRIzol reagent (Sigma, St Louis, MO, USA), and reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) and random primers (Toyobo, Tokyo, Japan) after treatment with RQ1 DNase I (Promega). Quantitative real–time PCR was performed using Go Taq qPCR Master Mix (Promega) in a Step One Plus (Applied Biosystems). Amplification conditions were: 94°C (5 min), followed by 40 cycles of 94°C (20 s), 55°C (20 s), and 72°C (50 s). All values were normalized to the expression level of Gapdh. The following primer sets were used: Enpp3: 5’-CTCATGCCCTGCACTACAGA-3’ and 5’-TAGCCTTTGGTTTGCTTGCT-3’, Gapdh: 5’-CCTCGTCCCGTAGACAAAATG-3’ and 5’-TCTCCACTTTGCCACTGCAA-3’, P2rx1: 5′-ACGAAACAAGAAGGTGGGAGT-3′ and 5′-AGGCCACTTGAGGTCTGGTAT-3′; P2rx2: 5′-GAGAGCTCCATCATCACCAAA-3′ and 5′-CAGGGTCTGGGAAGGAGTAAC-3′; P2rx3: 5′-CCGAGAACTTCACCATTTTCA-3′ and 5′-TTTATGTCCTTGTCGGTGAGG-3′; P2rx4: 5′-TGGCTACAATTTCAGGTTTGC-3′ and 5′-GATCATGGTTGGGATGATGTC-3′; P2rx5: 5′-AACCGTCTGGACAACAAACAC-3′ and 5′-TTTCATCAGGTCACGGAACTC-3′; P2rx6: 5′-CTCCTGGAGGTGGTTCATGTG -3′, 5’-GGCTTTGGCAAGCTTTACTTC-3’; P2rx7: 5’-TGTGTGCATTGACTTGCTCA-3’ and 5’-CTTGCAGACTTTTCCCAAGC-3’; Ifna1: 5’-TGGTCCTGGCGGTGATGAGC-3’ and 5’-AGGGATGGCTTGAGCCTTC-3’; Il12b: 5’-GGTTTGCCATCGTTTTGCTGG-3’ and 5’-CATCTTCTTCAGGCGTGTCAC-3’.
Generation of a monoclonal anti-mouse E-NPP3 antibody
Mouse E-NPP3-expressing NIH 3T3 cells were injected into the footpads of Enpp3-/- mice, and then popliteal lymph node cells were fused with P3-X63.Ag8.653 mouse myeloma cells. Hybridomas producing anti-mouse E-NPP3 antibodies were cloned by limiting dilution. An anti-mouse E-NPP3 antibody purified by affinity chromatography using protein G-sepharose (GE Healthcare) was labeled with Alexa Fluor 647 using an Alexa Fluor 647 monoclonal antibody labeling kit (Invitrogen).
Immunohistochemical staining
OCT compound (Leica)-embedded small intestine samples were sectioned and stained with the anti-mouse E-NPP3-Alexa Fluor 647 antibody. Images were obtained using an FV1000-D confocal microscope (Olympus).
Measurement of ATP
ATP concentrations in the small intestinal lumen were measured as described previously [31]. In brief, the mice were fasted overnight and then anesthetized with sevoflurane (Abbvie, North Chicago, IL, USA). The peritoneal cavity was opened and the small intestine was ligated with silk threads at every 3 cm. A total of 300 μl PBS was injected into each loop. At 15 min after injection, luminal fluid was collected, and the concentration of ATP in the fluid was measured with an ATP assay kit (Toyo Ink, Tokyo, Japan).
Mast cell reconstitution
To obtain bone marrow-derived mast cells, bone marrow cells from wild-type and Enpp3-/- mice were cultured with 5 ng/ml recombinant murine IL-3 (Pepro Tech) for 3 weeks. CD3-, CD4-, CD8-, B220-, FcεRI+ and c-kit+ bone marrow-derived mast cells were purified using a FACS Aria (BD Biosciences), and 2×106 mast cells were intravenously transferred into Enpp3-/- Kitw-sh/w-sh mice. At 2 and 6 days after the transfer, the number of mast cells in the small intestinal lamina propria was analyzed. Luminal ATP concentration was analyzed 6 days after the transfer.
Cell isolation
Isolated PPs from the small intestine were incubated in HBSS (Nacalai Tesque, Kyoto, Japan) containing 5 mM EDTA at 37°C for 15 min to remove epithelial cells. After washing with cold PBS, PPs and MLNs were chopped finely and incubated in RPMI 1640 (Nacalai Tesque) containing 10% fetal bovine serum (FBS) and 0.5 mg/ml collagenase D (Roche, Indianapolis, IN, USA) at 37°C for 20 min and then pass through a 40-μm Cell Strainer (BD Biosciences) to obtain single-cell suspensions. Small intestinal lamina propria lymphocytes were isolated using a previously described protocol [14,31].
Flow cytometry
Flow cytometric analysis was performed using a FACS Canto II flow cytometer (BD Biosciences) with FlowJo software (Tree Star, Ashland, OR, USA). pDCs and cDCs were isolated from MLNs, small intestinal lamina propria, PPs, bone marrow and spleens by using a FACS Aria (BD Biosciences). Instrumental settings were adjusted for four-color-stained samples.
Characterization of pDCs
Surface markers of pDCs were analyzed by flow cytometery. SILP cells gated on CD45+ PDCA-1+ CD11cmed were analyzed for expression of Siglec H, CCR9 and CD45RA. For cytokine production analysis, CD45+ PDCA-1+ CD11cmed pDCs were isolated from SILP of wild-type and Enpp3-/- mice by FACS Aria (BD Biosciences). The isolated pDCs were stimulated with or without CpG DNA (5μM) for 4 h. Expression of Ifna1 and Il12b was analyzed by quantitative RT-PCR.
Antibiotic treatment
Four-week-old mice were administrated combinations of four antibiotics including 1 mg/ml ampicillin (Nacalai Tesque), 1 mg/ml neomycin (Nacalai Tesque), 1 mg/ml metronidazole (Nacalai Tesque) and 500 μg/ml vancomycin (Duchefa Biochemie B.V.) in sterilized drinking water for 8 weeks.
Apoptosis assay
Isolated cells from MLNs were cultured in RPMI 1640 medium containing 10% FBS, 100 U/ml penicillin (Gibco, Grand Island, NE, USA), 100 μg/ml streptomycin (Gibco), 50 μM 2-mercaptoethanol (Gibco) and 20 ng/ml recombinant murine IL-4 (PeproTech, Rocky Hill, NJ) at 37°C with or without ATP (Sigma). After 3 h, the cells were washed in PBS and stained with annexin V and an anti-active caspase-3 antibody as well as PDCA-1 and CD11c antibodies. The frequencies of annexin V- and active caspase-3-positive cells among CD11cmed PDCA-1+ pDCs and CD11chigh cDCs were analyzed by flow cytometry. In some experiments, CD45+ PDCA-1+ and CD11cmed cells were isolated from MLN cells by a FACS Aria (BD Biosciences) and treated with ATP for 3 h, and then stained with annexin V and an anti-active caspase-3 antibody.
Induction of apoptosis in vivo
BALB/c mice were fasted overnight, anesthetized, and then the peritoneal cavity was opened. The proximal portion of the small intestine, which included PPs, was ligated with silk thread at every 3 cm. A total of 300 μl of 1 mM ATP-γS (Sigma) or PBS was injected into each loop, and then the peritoneal cavity was closed. After 4 h, PPs and small intestines were collected and cells were stained with anti-active caspase-3 antibody. The frequencies of active caspase-3-positive pDCs and cDCs in PPs were analyzed by flow cytometry.
Statistical analysis
Comparisons between two groups were performed by Student’s t-test. Differences between multiple groups were analyzed using Tukey’s test followed by one-way ANOVA with JMP Pro 12 software. Differences with P < 0.05 were considered to be significant. Results are expressed as the mean ± SD.
Results
High expression of Enpp3 in small intestinal epithelia
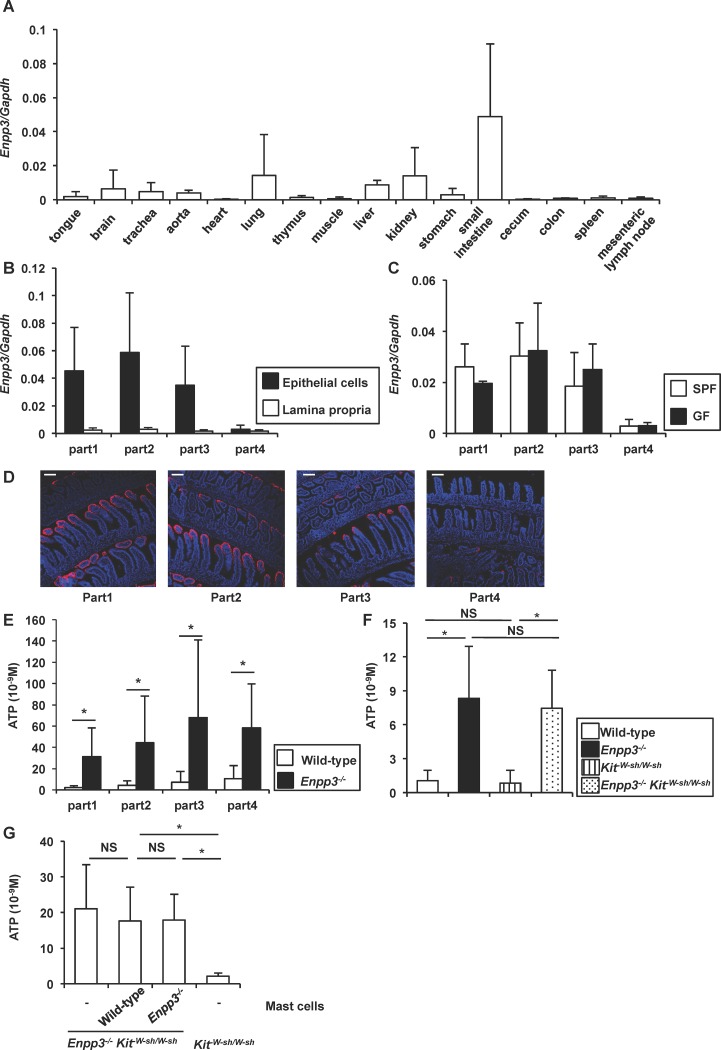
Enpp3 is highly expressed on epithelia of the human retina and endometrium as well as activated basophils and mast cells [10,39,40]. Therefore, we analyzed expression of Enpp3 in mouse tissues by quantitative RT-PCR. Enpp3 was highly expressed in the small intestine (Fig 1A). In the small intestine, Enpp3 was expressed at a higher level in the epithelial layer than in the lamina propria (Fig 1B). Enpp3 expression was highly observed in the proximal portion of the small intestinal epithelia (Fig 1B). Enpp3 expression was not reduced in germ-free mice, in which intestinal microbiota was absent, suggesting that Enpp3 expression is not dependent on intestinal bacteria (Fig 1C). We also analyzed protein expression of E-NPP3 in the small intestine by immunohistochemistry (Fig 1D). Similar to the Enpp3 mRNA expression pattern, protein expression of E-NPP3 was higher in the proximal portion than that in the distal portion of the small intestine. In addition, E-NPP3 was expressed in the epithelial layer at the tip of villi in the small intestine. Therefore, we determined whether the ATP-hydrolyzing ectoenzyme E-NPP3 regulates the ATP concentration in the small intestinal lumen using Enpp3-/- mice. Ligated intestinal loops of four parts from proximal to distal regions of the small intestine were created, and then PBS was injected into the loop. After 15 min, the concentration of ATP was analyzed in the luminal content. ATP concentrations in the lumens of all parts were elevated in the small intestine of Enpp3-/- mice (Fig 1E). Because E-NPP3 is highly expressed in activated mast cells, which are abundant in the intestinal lamina propria [10], we investigated whether E-NPP3 on mast cells contributes to controlling luminal ATP by crossing Enpp3-/- mice with KitW-sh/W-sh mice that lack mast cells. In Enpp3-/- KitW-sh/W-sh mice, the luminal ATP concentration in the small intestine was increased to a similar level as that in Enpp3-/- mice (Fig 1F). We then transferred wild-type or Enpp3-/- bone marrow-derived mast cells into Enpp3-/- KitW-sh/W-sh mice, and analyzed luminal ATP concentration (Fig 1G). Transferred bone marrow-derived mast cells were present in the small intestinal lamina propria at 2 days after the transfer (S1 Fig). Adoptive transfer of wild-type mast cells did not decrease the luminal ATP concentration in Enpp3-/- KitW-sh/W-sh mice at 6 days after the transfer, demonstrating that E-NPP3 on mast cells does not control the ATP concentration in the intestinal lumen. These findings indicate that E-NPP3 is highly expressed on the epithelial layer of the small intestine and controls the luminal ATP concentration.
Fig 1. Expression of Enpp3 in small intestinal epithelia.
(A) Quantitative RT-PCR analysis of Enpp3 mRNA expression in the indicated tissues (n = 3). (B) Quantitative RT-PCR analysis of Enpp3 mRNA expression in epithelial cells and lamina propria in four parts of the small intestine. A smaller number represents a more proximal portion of the intestine (n = 4). (C) Quantitative RT-PCR analysis of Enpp3 mRNA expression in epithelial cells of the small intestine in specific-pathogen free (SPF) and germ-free (GF) mice (n = 3). (D) Immunohistochemical analysis of the small intestine. E-NPP3 (red) and DAPI (blue). Swiss roll frozen sections were stained with the anti-mouse E-NPP3 antibody. A smaller number represents more proximal portion of the intestine. Scale bars, 100 μm. (E) ATP concentrations in luminal contents of the small intestine of wild-type and Enpp3-/- mice. All data are mean values ± SD (n = 6 per groups). *p < 0.05. (F) ATP concentration in the proximal portion of the small intestinal lumen from wild-type, Enpp3-/-, KitW-sh/W-sh and Enpp3-/- KitW-sh/W-sh mice. All data are mean values ± SD (n = 4 per groups). *p < 0.05, NS: not significant. (G) ATP concentration in the proximal portion of the small intestinal lumen from KitW-sh/W-sh and Enpp3-/- KitW-sh/W-sh mice with or without adoptive transfer of wild-type or Enpp3-/- bone marrow-derived mast cells. All data are mean values ± SD (n = 5 for mast cells transferred mice groups and KitW-sh/W-sh mice group, and n = 3 for non transferred Enpp3-/- KitW-sh/W-sh mice group). *p < 0.05, NS: not significant.
Reduced number of pDCs in Enpp3-/- intestines
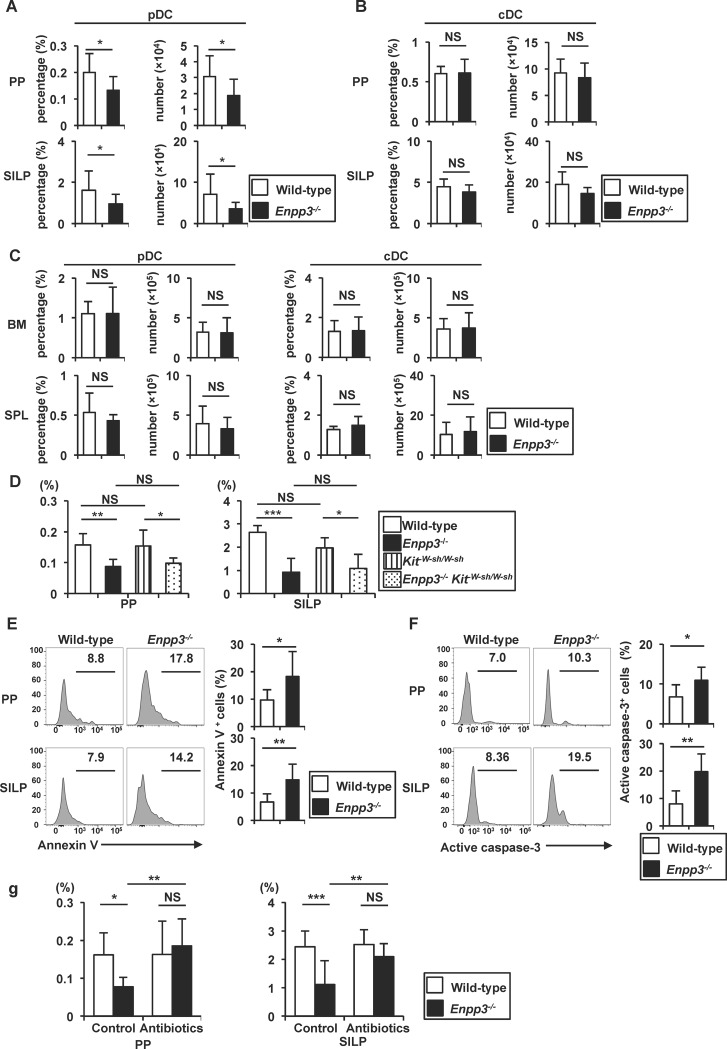
Luminal ATP induces differentiation of IL-17-producing T cells through activation of a unique subtype of lamia propria DCs [14]. Furthermore, Entpd7-/- mice show increased numbers of IL-17-producing T cells in the small intestinal lamia propria (SILP) with an elevated luminal ATP concentration [31]. Therefore, we analyzed the number of IL-17-producing T cells and other immune cells in the intestines of Enpp3-/- mice. The numbers of B220+ cells and CD4+ cells were not altered in the SILP and PPs of Enpp3-/- mice (S2A Fig). However, the number of IL-17-producing T cells was slightly increased in the SILP of Enpp3-/- mice (S2B Fig). Although ATP has been shown to control follicular T helper (Tfh) cell numbers in PPs [21], the frequency and number of Tfh cells was not altered in PPs of Enpp3-/- mice (S2C Fig). We also analyzed other immune cell populations, such as pDCs and conventional DCs (cDCs). The frequency and number of CD11cmed PDCA-1+ pDCs, but not CD11chigh cDCs, were decreased in the SILP and PPs of Enpp3-/- mice compared with wild-type mice (Fig 2A and 2B and S3A Fig). We next analyzed the numbers of pDCs and cDCs in the bone marrow and spleen (Fig 2C and S3B Fig). The frequency and number of CD11cmed PDCA-1+ pDCs and CD11chigh cDCs were not altered in the bone marrow and spleen of Enpp3-/- mice. The number of pDCs in the SILP and PPs was also reduced in Enpp3-/- KitW-sh/W-sh mice (Fig 2D). Thus, the number of pDCs was decreased selectively in the intestines of Enpp3-/- mice.
Fig 2. Decrease in the number of intestinal pDCs in Enpp3-/- mice.
(A–C) Frequency and number of CD45+ PDCA-1+ CD11cmed pDCs (A, C) and CD45+ PDCA-1- CD11c high cDCs (B, C) in the Peyer’s patches (PPs), small intestinal lamina propria (SILP), bone marrow (BM), and spleen (SPL) of wild-type and Enpp3-/- mice. All data are mean values ± SD (n = 7 for PP and SILP, and n = 6 for SPL and BM). *p < 0.05, NS: not significant. (D) Frequency of CD45+ PDCA-1+ CD11cmed pDCs in the PPs and SILP of wild-type (n = 6), Enpp3-/- (n = 8), KitW-sh/W-sh (n = 7), and Enpp3-/- KitW-sh/W-sh (n = 6) mice. All data are mean values ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant. (E, F) Frequency of annexin V-positive (E) and active caspase-3-positive (F) cells gated on CD45+ PDCA-1+ CD11cmed pDCs from the PPs and SILP of wild-type and Enpp3-/- mice. Representative histograms are shown (left) and the means ± SD of the percentages of positive cells (right) are shown (n = 6 in e, and n = 5 in f). *p < 0.05, **p < 0.01. (G) Frequency of PDCA-1+ CD11cmed pDCs in the PPs and SILP from antibiotic-treated wild-type (n = 11) and Enpp3-/- (n = 12) mice as well as untreated wild-type (n = 10) and Enpp3-/- (n = 10) mice. Data are the means ± SD of the percentages of pDCs. *p < 0.05, **p < 0.01, ***p < 0.001, NS: not significant.
We next analyzed the function of intestinal pDCs of Enpp3-/- mice. Surface expression of pDC markers in pDCs in SILP was first analyzed by flow cytometry (S4A Fig). Expression of Siglec H, which is selectively expressed in pDCs, was not altered in Enpp3-/- pDCs. CCR9, which was highly expressed in pDC migrating into the small intestine, was also normally expressed in Enpp3-/- pDCs. CD45RA, which was expressed higher in pDCs than cDCs, was not reduced in Enpp3-/- pDCs. We then analyzed cytokine production from SILP pDCs. pDCs were isolated from SILP of wild type and Enpp3-/- mice, stimulated with CpG DNA, and analyzed for expression of Ifna1 (encoding IFN-α) and Il12b (encoding IL-12p40) by quantitative RT-PCR (S4B Fig). Enpp3-/- pDCs showed normal expression of these cytokines. Thus, the functions of pDCs in the small intestine of Enpp3-/- mice were not impaired.
We next analyzed whether intestinal pDCs of Enpp3-/- mice were sensitive to cell death by staining with annexin V (Fig 2E). Annexin V-positive pDCs was increased in PPs and SILP of Enpp3-/- mice compared with wild-type mice. We also stained pDCs with an antibody that detects active caspase-3 produced during the process of apoptosis (Fig 2F). There were increased numbers of pDCs positive for active caspase-3 in PPs and SILP of Enpp3-/- mice compared with wild-type mice. These findings indicate a decrease of intestinal pDCs due to the enhanced apoptosis in Enpp3-/- mice. Because the intestinal ATP concentration increases in a commensal microbiota-dependent manner [14], we examined whether commensal microbiota contribute to the reduction of intestinal pDC numbers (Fig 2G and S3C Fig). Wild-type and Enpp3-/- mice were orally treated with combinations of four antibiotics (vancomycin, streptomycin, metronidazole, and ampicillin) for 8 weeks. In antibiotic-treated Enpp3-/- mice, the numbers of pDCs in the SILP and PPs were increased compared with those in untreated Enpp3-/- mice. Taken together, these findings indicate that intestinal pDCs decrease because of the increase in the concentration of intestinal ATP, which was dependent on commensal microbiota in the absence of E-NPP3.
Induction of pDC apoptosis by extracellular ATP
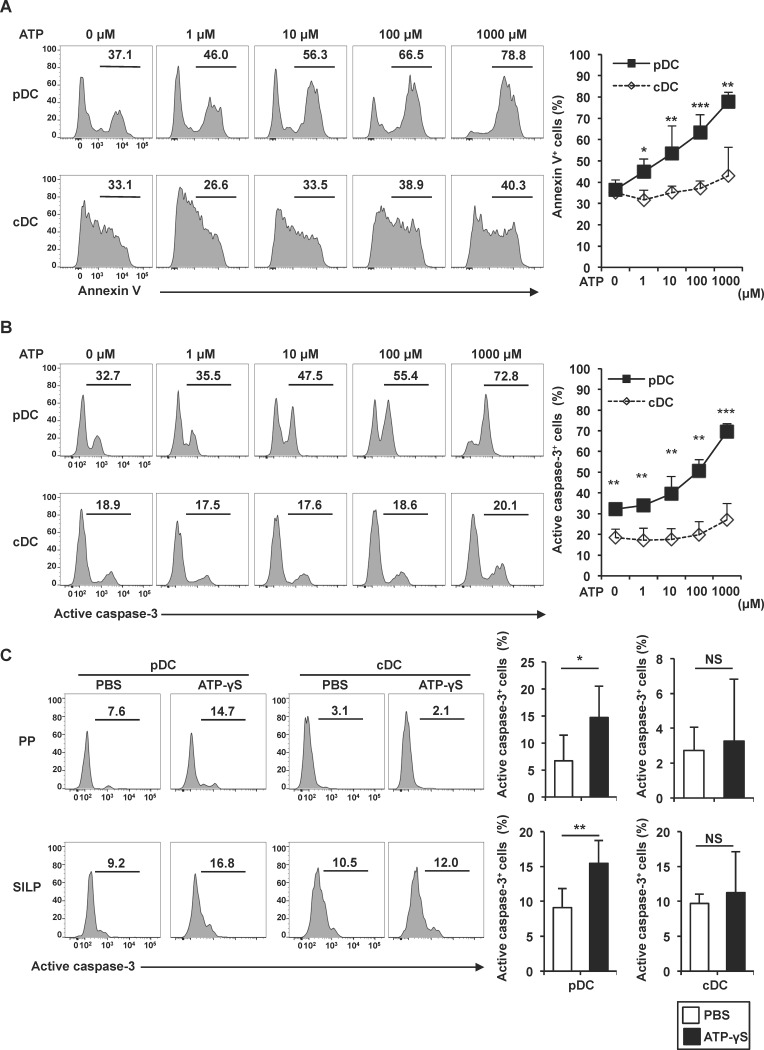
We next analyzed the mechanisms responsible for the reduced number of pDCs in the intestine. Several studies have indicated that extracellular ATP induces cell death in many cell types including monocytes/macrophages, DCs, and T cells [15,18,19,21,41]. Therefore, we determined whether extracellular ATP induces cell death of pDCs. MLN cells were isolated, treated with increasing concentrations of ATP for 3 h, and analyzed for annexin V binding by flow cytometry in pDC and cDC populations (Fig 3A). ATP did not increase annexin V-positive cDCs, but increased annexin V binding to pDCs in a dose-dependent manner. Then, the cells were analyzed for expression of active caspase-3 (Fig 3B). ATP treatment showed dose-dependent enhancement of active caspase-3 in pDCs, but not in cDCs. These results indicate that pDCs are highly sensitive to ATP-induced cell death. We next analyzed the in vivo effect of ATP on apoptosis of intestinal pDCs. Non-hydrolyzable ATP, ATP-γS, was administered directly into the small intestine. At 4 h after administration, pDCs and cDCs in PPs and SILP were analyzed for expression of active caspase-3 (Fig 3C). Luminal administration of ATP-γS increased active caspase-3 in pDCs, but not in cDCs. Thus, ATP induced cell death of intestinal pDCs in vitro and in vivo.
Fig 3. Enhancement of ATP-induced apoptosis of pDCs.
(A, B) Cells isolated from MLNs were treated with the indicated concentrations of ATP for 3 h. Annexin V-positive cells (A) and active caspase-3-positive cells (B) among PDCA-1+ CD11cmed pDCs and PDCA-1- CD11chigh cDCs were analyzed by flow cytometry. Representative histograms are shown (left) and the means ± SD (n = 3) of the percentages of annexin V-positive (A) and active caspase-3-positive cells (B) are shown (right). *p < 0.05, **p < 0.01, ***p < 0.001. (C) Frequency of active caspase-3-positive cells among CD45+ PDCA-1+ CD11cmed pDCs and and PDCA-1- CD11chigh cDCs from the PPs and SILP of mice injected with 300 μl PBS (n = 6) or 1 mM ATP-γS (n = 5) into their small intestinal lumen. Representative histograms are shown (left) and the means ± SD of the percentages of positive cells are shown (right). *p < 0.05, **p < 0.01, NS: not significant.
P2X7 mediates ATP-induced pDC cell death
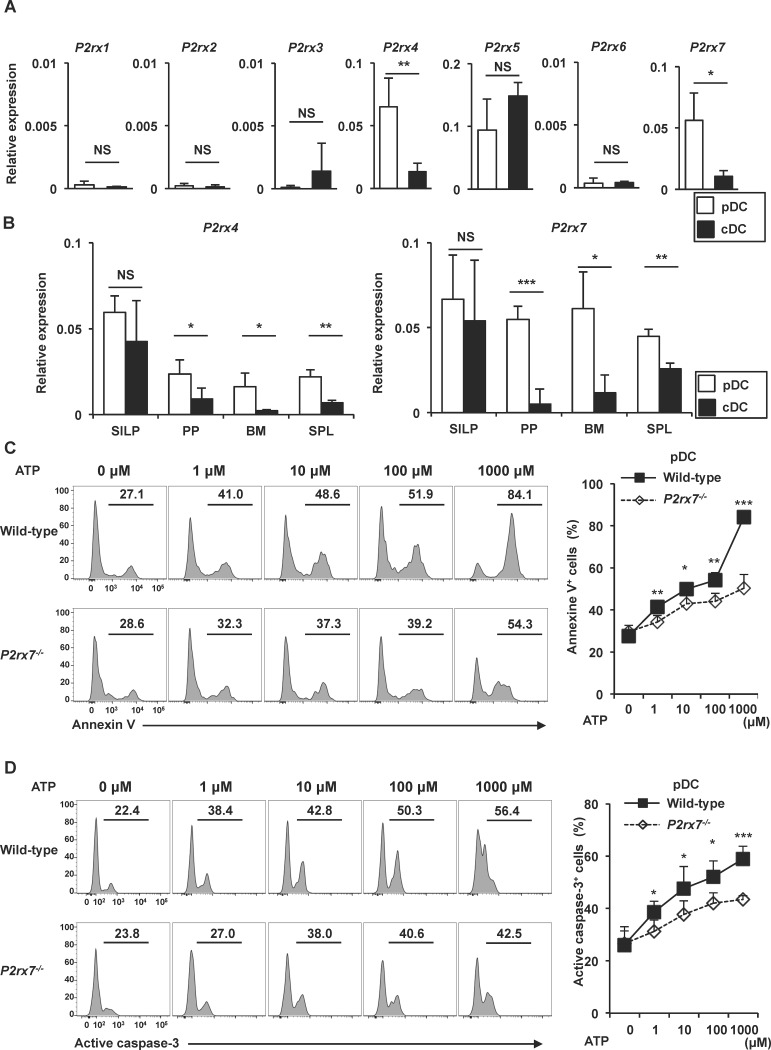
We next analyzed the mechanisms by which intestinal pDCs are highly sensitive to ATP-induced cell death. The ATP sensors, P2X4 and P2X7, are involved in ATP-induced cell death of many cell types [19,41–43]. Therefore, we analyzed mRNA expression of P2X receptors in pDCs and cDCs of MLN by quantitative RT-PCR (Fig 4A). pDCs expressed higher level of P2rx4 and P2rx7 than cDCs. We next compared expression levels of P2rx4 and P2rx7 in pDCs and cDCs of several tissues, such as SILP, PP, bone marrow, and spleen (Fig 4B). In accordance with the fact that ATP activates intestinal cDCs [14], both pDCs and cDCs in SILP similarly and highly expressed P2rx4 and P2rx7. In all the tissues analyzed (SILP, PP, MLN, bone marrow and spleen), high expression of P2rx7 in pDCs, but not cDCs, was observed. In contrast, expression level of P2rx4 in pDCs was not so high in PP, bone marrow, and spleen, although it was expressed at high level in MLN and SILP. Therefore, we focused on P2X7, and we examined ATP-induced cell death of pDCs in P2rx7-/- mice. pDCs were isolated from MLN of wild-type and P2rx7-/- mice, treated with the indicated concentrations of ATP for 3 h, and then analyzed for annexin V binding and active caspase-3 in the pDC population (Fig 4C and 4D). ATP treatment enhanced annexin V binding and active caspase-3 in wild-type pDCs in a dose-dependent manner. However, the ATP-dependent increased of annexin V binding- and active caspase-3-positive cells were reduced in P2rx7-/- pDCs compared to those in wild-type cells. These findings indicate that ATP induces cell death of pDCs via P2X7.
Fig 4. P2X7-dependent induction of pDC apoptosis in vitro.
(A) Expression of P2X receptors in pDCs or cDCs of MLN was analyzed by quantitative RT-PCR. Data are means ± SD (n = 3). *p < 0.05, **p < 0.01. (B) mRNA expression of P2rx4 and P2rx7 in pDCs and cDCs of SILP, PP, bone marrow (BM) and spleen (SPL) was analyzed by quantitative RT-PCR. Data are means ± SD (n = 3). *p < 0.05, **p < 0.01. (C, D) Isolated MLN pDCs of wild-type and P2rx7-/- mice were treated with the indicated concentrations of ATP for 3 h. Annexin V-positive cells (C) and active caspase-3-positive cells (D) were analyzed by flow cytometry. Representative histograms are shown (left) and the means ± SD of the percentages of annexin V-positive (C) and active caspase-3-positive cells (D) (n = 4) are shown (right). *p < 0.05, **p < 0.01, ***p < 0.001.
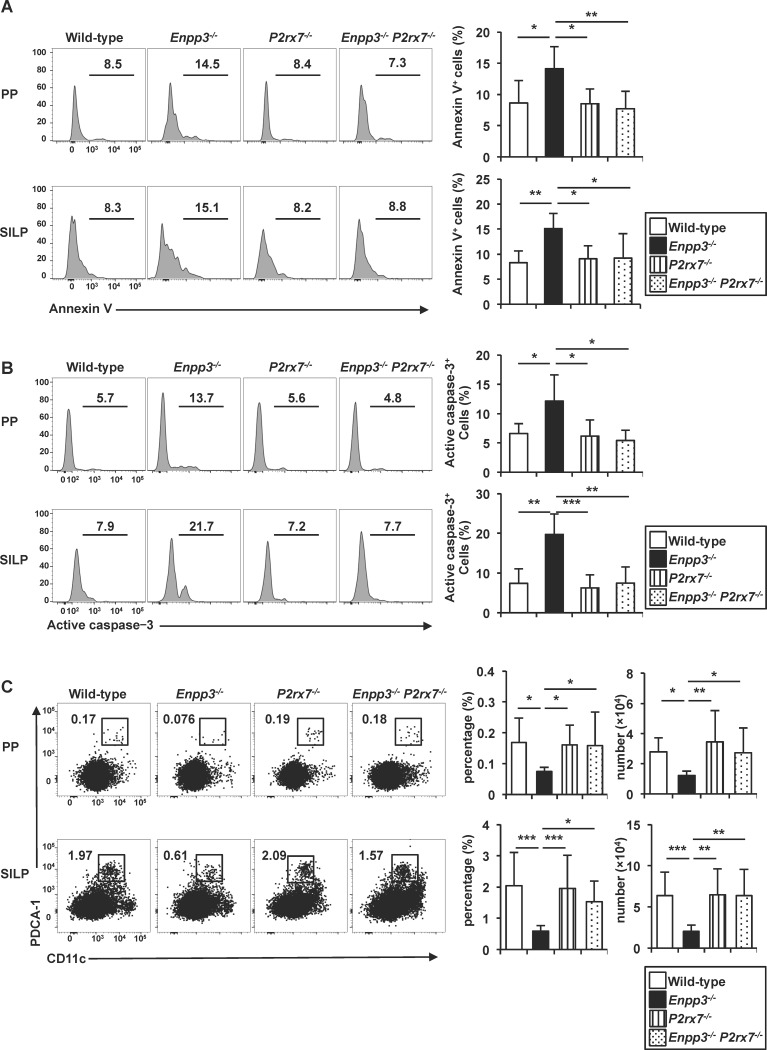
We next analyzed the in vivo role of P2X7 in the ATP-induced cell death of pDCs by inducing P2rx7 deficiency in Enpp3-/- mice. In Enpp3-/- P2rx7-/- mice, the numbers of annexin V- and active caspase-3-positive pDCs in PPs and SILP were dramatically reduced compared with those in Enpp3-/- mice (Fig 5A and 5B). Accordingly, the numbers of pDCs in the PPs and SILP of Enpp3-/- P2rx7-/- mice were increased to comparable levels to those in wild-type mice (Fig 5C). Taken together, these findings suggest that P2X7 mediates the ATP-dependent cell death of intestinal pDCs.
Fig 5. P2X7-dependent induction of pDC apoptosis in vivo.
(A, B) Frequencies of annexin V-positive (A) and active caspase-3-positive (B) cells gated on CD45+ PDCA-1+ CD11cmed pDCs from the PPs and SILP of wild-type, Enpp3-/-, P2rx7-/-, and Enpp3-/- P2rx7-/- mice (n = 7 per groups in a, and n = 5 per groups in b). Representative histograms are shown (left) and the means ± SD of the percentages of annexin V-positive (A) and active caspase-3-positive cells (B) are shown (right). *p < 0.05, **p < 0.01 (c) Frequency and number of PDCA-1+ CD11cmed pDCs in the PPs and SILP from wild-type, Enpp3-/-, P2rx7-/-, and Enpp3-/- P2rx7-/- (n = 13 per groups) mice. Representative dot plots are shown (left) and the means ± SD of the percentages of pDCs are shown (right). *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
E-NPP3 (also known as CD203c) regulates basophil- and mast cell-mediated chronic allergic inflammation [10]. In the present study, we demonstrate that E-NPP3 is highly expressed on small intestinal epithelial cells and regulates the luminal ATP concentration. In Enpp3-/- mice, the number of intestinal pDCs was decreased, which was accompanied by an increased concentration of luminal ATP. Intestinal pDCs were highly sensitive to ATP-induced cell death. Thus, E-NPP3 is responsible for the maintenance of pDCs via the control of ATP concentrations in the small intestine.
Extracellular ATP induces immune responses by acting on different immune cell types via P2 receptors [1,2,9,44]. Conversely, several lines of evidence indicate that ATP induces cell death of several immune cell types to modulate immune responses [15–21]. The present study demonstrates that intestinal pDCs are highly sensitive to ATP-induced cell death. In the absence of P2X7, ATP-induced pDC cell death was severely inhibited. However, high concentration of ATP still induced pDC death of P2rx7-/- mice. In this regard, P2rx4, which was also highly expressed in pDCs, might contribute to the ATP-induced cell death. P2rx7 was also highly expressed in pDCs in other tissues such as spleen and bone marrow. Thus, P2X7 can be used as a general marker for pDCs. In addition, in a situation when extracellular ATP increases in those tissues, pDCs might decrease in number. Another intriguing point is that P2rx4 and P2rx7 were similarly expressed in pDCs and cDCs in SILP. Thus, cDCs in SILP were supposed to respond to ATP via P2X4 and P2X7, but showed no ATP-induced apoptotic response. In this regard, a subset of cDCs in the intestinal lamina propria showed ATP-induced gene expression [14]. Thus, pDCs and cDCs in SILP might possess differential properties in terms of ATP-induced responses, apoptosis and gene expression, respectively. It would be an interesting future issue to analyze ATP-induced activation of signaling pathways in pDCs and cDCs in SILP.
It is well known that pDCs play essential roles in host defense against viral infection through high production of type I IFNs [32,45]. Accordingly, chronic viral infection has been shown to cause decreases in the pDC number and type I IFN production in mice [46]. A decreased number of pDCs has also been found in patients with chronic infection by hepatitis B or C viruses [47,48]. Cell death of pDCs during viral infection has been shown to be mediated by type I IFN [49]. Thus, pDC numbers are tightly regulated during viral infections. The present study demonstrates that the numbers of intestinal pDCs are controlled by extracellular ATP. In the case of intestinal pDCs, cell death was directly induced by ATP, and did not appear to be dependent on autocrine type I IFNs based on the following two findings. First, an alteration in the number of pDCs in gut-associated lymphoid tissue has not been described in Ifnar1-/- mice that lack type I IFN signaling [38]. Second, our unpublished results showed that mRNA levels of genes encoding type IFNs (Ifna and Ifnb) were not elevated in PPs or small intestinal tissues of Enpp3-/- mice.
In addition to the host defense against viral infections, pDCs have been implicated in regulation of several immune responses, including T cell-independent induction of IgA-producing plasma cells and induction of oral tolerance [38] [50]. Accordingly, the number of IgA-expressing plasma cells in SILP was reduced in Enpp3-/- mice (our unpublished results). Induction of oral tolerance was also partially reduced in Enpp3-/- mice (our unpublished results). Although it remains unknown whether these impairments were due to the reduced number of intestinal pDCs, the deficiency of Enpp3 leads to defective intestinal immune responses.
Considering that the intestinal mucosa is one of main routes for viral invasion, the regulation of pDCs by ATP is supposed to be associated with the host–virus interaction in the intestines. Several lines of evidence indicate that increased ATP production by host cells enhances viral growth [51,52]. Extracellular ATP also mediates infection and budding of human immunodeficiency virus 1 [53,54]. Thus, ATP in the extracellular and intracellular compartments is used by viruses for their propagation in the host. In addition, ATP increases cell death of intestinal pDCs, a major player in anti-viral responses. Therefore, viruses might promote ATP production by host cells for evasion of anti-viral immune responses induced by pDCs as well as promotion of viral growth in the intestines. The host, in turn, expresses E-NPP3 at the mucosal surface to decrease the concentration of ATP that might be harmful when produced in excess.
Tfh cells in PPs have been shown to be sensitive to P2X7-mediated cell death [21]. However, in the present study, the number of Tfh cells in PPs was not decreased in Enpp3-/- mice. In this regard, it might be possible that both pDCs and Tfh cells are sensitive to ATP, but pDCs exhibit higher sensitivity to ATP than Tfh cells. Indeed, Tfh cells become resistant to ATP-mediated cell death by TCR stimulation [21]. Furthermore, a high concentration of ATP (over 1 mM) is required to induce Tfh cell death [21], whereas the micromolar levels of ATP induced cell death of intestinal pDCs.
Nucleotide-converting ectoenzymes, such as E-NTPD1, E-NTPD7, and E-NPP3 regulate ATP-mediated immune and inflammatory responses via ATP hydrolysis [10,31,55–57]. Thus, many studies have focused on negative regulation of immune responses through control of the extracellular ATP concentration. The present study demonstrates another aspect of the importance of the control of ATP that induces cell death and thus promotes disadvantageous conditions in terms of the maintenance of intestinal homeostasis. E-NPP3 expressed on the small intestinal epithelial cell layer controls the intestinal immune response via the prevention of pDC death in the intestines.
In the present study, we revealed that the ectoenzyme E-NPP3 regulates the number of small intestinal pDCs, which are involved in immune responses in the intestine. However, Th17 cell numbers in the small intestine also increased in Enpp3-/- mice. A previous study has demonstrated that another ectoenzyme, E-NTPD7, which was also highly expressed on the epithelial cells of the small intestine, regulates Th17 cell responses [31]. It is possible that other ATP-hydrolyzing ectoenzymes are expressed on intestinal epithelial cell layers. Therefore, it would be an interesting future study to analyze the role of these ectoenzymes in the control of pDC numbers in the intestines. Elucidation of the role of the family of ATP-hydrolyzing ectoenzymes in the regulation of immune responses might lead to development of a novel strategy to modulate intestinal inflammation, such as inflammatory bowel diseases.
Supporting information
Bone marrow-derived mast cells were transferred into KitW-sh/W-sh mice. At day 2 and day 6 after the reconstitution, CD3- CD4- CD8- B220- cells were gated and frequencies of mast cells in the small intestine were analyzed for expression of c-kit+ FcεRI+ by flow cytometory.
(TIFF)
(A) Frequency and number of CD4+ T cells and B220+ B cells in the PPs and SILP of wild-type (n = 6) and Enpp3-/- (n = 6) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of CD4+ or B220+ cells are shown (right). NS: not significant. (B) Frequency and number of IL-17-producing CD4+ T cells in the small intestine of wild-type (n = 5) and Enpp3-/- (n = 5) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of IL-17+ CD4+ cells are shown (right). *p < 0.05, NS: not significant. (C) Frequency and numbers of CD4+ ICOS+ CXCR5+ follicular helper T (Tfh) cells in the PPs of wild-type (n = 5) and Enpp3-/- (n = 5) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of Tfh cells are shown (right). NS: not significant.
(TIFF)
(A, B) Frequency of CD45+ PDCA-1+ CD11cint pDCs and CD45+ PDCA-1- CD11c high cDCs in the PPs, SILP (A), BM, and SPL (B) of wild-type and Enpp3-/- mice. Representative dot plots are shown. Numbers in dot plots indicate the percentages of cells in the respective areas. (C) Frequency of PDCA-1+ CD11cint pDCs in the PPs and SILP from antibiotic-treated wild-type (n = 11) and Enpp3-/- (n = 12) mice or untreated wild-type (n = 10) and Enpp3-/- (n = 10) mice. Representative dot plots are shown. Numbers in dot plots indicate the percentages of cells in the respective areas.
(TIFF)
(A) Surface expression of Siglec H, CCR9 and CD45RA on CD45+ PDCA-1+ CD11cmed pDCs from SILP analyzed by flow cytometry. (B) CD45+ PDCA-1+ CD11cmed pDCs were isolated from SILP of wild-type and Enpp3-/- mice with FACS Aria. pDCs were stimulated with CpG DNA (5 μM) for 4 h. Expression of Ifna1 and Il12b was analyzed by quantitative RT-PCR (n = 3). NS: not significant.
(TIFF)
Acknowledgments
We thank H. Suto for providing KitW-sh/W-sh mice, T. Kondo and Y. Magota for technical assistance, and C. Hidaka for secretarial assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support was provided by: the Ministry of Education, Culture, Sports, Science and Technology of Japan, Japan Science and Technology Agency (Recipient: Kiyoshi Takeda, T15K15152, A15H02511); Japan Agency for Medical Research and Development (Recipient: Kiyoshi Takeda, 15gm011002h0006); and Mochida Memorial Foundation for Medical and Pharmaceutical (Recipient: Shih-Han Tsai).
References
- 1.Di Virgilio F (2005) Purinergic mechanism in the immune system: A signal of danger for dendritic cells. Purinergic Signal 1: 205–209. 10.1007/s11302-005-6312-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, et al. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286. 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trautmann A (2009) Extracellular ATP in the immune system: more than just a "danger signal". Sci Signal 2: pe6 10.1126/scisignal.256pe6 [DOI] [PubMed] [Google Scholar]
- 4.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, et al. (2001) The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol 166: 7172–7177. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, et al. (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159: 1451–1458. [PubMed] [Google Scholar]
- 6.Ferrari D, La Sala A, Chiozzi P, Morelli A, Falzoni S, et al. (2000) The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J 14: 2466–2476. 10.1096/fj.00-0031com [DOI] [PubMed] [Google Scholar]
- 7.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, et al. (2008) Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1: ra6 10.1126/scisignal.1160583 [DOI] [PubMed] [Google Scholar]
- 8.Padeh S, Cohen A, Roifman CM (1991) ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol 146: 1626–1632. [PubMed] [Google Scholar]
- 9.Bulanova E, Bulfone-Paus S (2010) P2 receptor-mediated signaling in mast cell biology. Purinergic Signal 6: 3–17. 10.1007/s11302-009-9173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai SH, Kinoshita M, Kusu T, Kayama H, Okumura R, et al. (2015) The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 42: 279–293. 10.1016/j.immuni.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 11.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, et al. (2011) ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal 4: ra12 10.1126/scisignal.2001270 [DOI] [PubMed] [Google Scholar]
- 12.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, et al. (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919. 10.1038/nm1617 [DOI] [PubMed] [Google Scholar]
- 13.Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, et al. (2012) Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun 3: 1034 10.1038/ncomms2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, et al. (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455: 808–812. 10.1038/nature07240 [DOI] [PubMed] [Google Scholar]
- 15.Placido R, Auricchio G, Falzoni S, Battistini L, Colizzi V, et al. (2006) P2X(7) purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell Immunol 244: 10–18. 10.1016/j.cellimm.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Lee S, Wu D, Yeh J, Ellamushi H, et al. (2013) P2X7 purinoceptors contribute to the death of Schwann cells transplanted into the spinal cord. Cell Death Dis 4: e829 10.1038/cddis.2013.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen LT, Knowles AF (2003) Extracellular ATP and adenosine induce cell apoptosis of human hepatoma Li-7A cells via the A3 adenosine receptor. Br J Pharmacol 140: 1009–1018. 10.1038/sj.bjp.0705523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD (1991) Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 112: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, et al. (1998) Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 275: F962–971. [DOI] [PubMed] [Google Scholar]
- 20.Lepine S, Le Stunff H, Lakatos B, Sulpice JC, Giraud F (2006) ATP-induced apoptosis of thymocytes is mediated by activation of P2 X 7 receptor and involves de novo ceramide synthesis and mitochondria. Biochim Biophys Acta 1761: 73–82. 10.1016/j.bbalip.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, et al. (2014) ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity 41: 789–801. 10.1016/j.immuni.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694. 10.1016/j.bbamcr.2008.01.024 [DOI] [PubMed] [Google Scholar]
- 23.Schetinger MR, Morsch VM, Bonan CD, Wyse AT (2007) NTPDase and 5'-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 31: 77–98. [DOI] [PubMed] [Google Scholar]
- 24.Robson SC, Sevigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430. 10.1007/s11302-006-9003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goding JW, Grobben B, Slegers H (2003) Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta 1638: 1–19. [DOI] [PubMed] [Google Scholar]
- 26.Stefan C, Jansen S, Bollen M (2005) NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci 30: 542–550. 10.1016/j.tibs.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Stefan C, Jansen S, Bollen M (2006) Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal 2: 361–370. 10.1007/s11302-005-5303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, et al. (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110: 1225–1232. 10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- 29.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, et al. (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204: 1257–1265. 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schena F, Volpi S, Faliti CE, Penco F, Santi S, et al. (2013) Dependence of immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep 3: 1824–1831. 10.1016/j.celrep.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 31.Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, et al. (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 190: 774–783. 10.4049/jimmunol.1103067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, et al. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2: 1144–1150. 10.1038/ni736 [DOI] [PubMed] [Google Scholar]
- 33.Colonna M, Trinchieri G, Liu YJ (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5: 1219–1226. 10.1038/ni1141 [DOI] [PubMed] [Google Scholar]
- 34.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, et al. (2007) CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A 104: 6347–6352. 10.1073/pnas.0609180104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, et al. (2008) CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol 9: 1253–1260. 10.1038/ni.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuno S, Kanai T, Mikami Y, Sujino T, Ono Y, et al. (2012) CCR9+ plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol Lett 146: 64–69. 10.1016/j.imlet.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL (2007) Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol 179: 2690–2694. [DOI] [PubMed] [Google Scholar]
- 38.Tezuka H, Abe Y, Asano J, Sato T, Liu J, et al. (2011) Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34: 247–257. 10.1016/j.immuni.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 39.Hood BL, Liu B, Alkhas A, Shoji Y, Challa R, et al. (2015) Proteomics of the human endometrial glandular epithelium and stroma from the proliferative and secretory phases of the menstrual cycle. Biol Reprod 92: 106 10.1095/biolreprod.114.127217 [DOI] [PubMed] [Google Scholar]
- 40.Reigada D, Lu W, Zhang X, Friedman C, Pendrak K, et al. (2005) Degradation of extracellular ATP by the retinal pigment epithelium. Am J Physiol Cell Physiol 289: C617–624. 10.1152/ajpcell.00542.2004 [DOI] [PubMed] [Google Scholar]
- 41.Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, et al. (1999) P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol 276: C1139–1147. [DOI] [PubMed] [Google Scholar]
- 42.Jun DJ, Kim J, Jung SY, Song R, Noh JH, et al. (2007) Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem 282: 37350–37358. 10.1074/jbc.M707915200 [DOI] [PubMed] [Google Scholar]
- 43.Solini A, Santini E, Chimenti D, Chiozzi P, Pratesi F, et al. (2007) Multiple P2X receptors are involved in the modulation of apoptosis in human mesangial cells: evidence for a role of P2X4. Am J Physiol Renal Physiol 292: F1537–1547. 10.1152/ajprenal.00440.2006 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, et al. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795. 10.1126/science.1132559 [DOI] [PubMed] [Google Scholar]
- 45.Liu YJ (2005) IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23: 275–306. 10.1146/annurev.immunol.23.021704.115633 [DOI] [PubMed] [Google Scholar]
- 46.Lee LN, Burke S, Montoya M, Borrow P (2009) Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol 182: 7178–7189. 10.4049/jimmunol.0802526 [DOI] [PubMed] [Google Scholar]
- 47.Duan XZ, Wang M, Li HW, Zhuang H, Xu D, et al. (2004) Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol 24: 637–646. 10.1007/s10875-004-6249-y [DOI] [PubMed] [Google Scholar]
- 48.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, et al. (2004) Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis 190: 1919–1926. 10.1086/425425 [DOI] [PubMed] [Google Scholar]
- 49.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, et al. (2011) Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med 208: 2367–2374. 10.1084/jem.20110654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, et al. (2008) Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29: 464–475. 10.1016/j.immuni.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang CW, Li HC, Hsu CF, Chang CY, Lo SY (2009) Increased ATP generation in the host cell is required for efficient vaccinia virus production. J Biomed Sci 16: 80 10.1186/1423-0127-16-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui EK, Nayak DP (2001) Role of ATP in influenza virus budding. Virology 290: 329–341. 10.1006/viro.2001.1181 [DOI] [PubMed] [Google Scholar]
- 53.Seror C, Melki MT, Subra F, Raza SQ, Bras M, et al. (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med 208: 1823–1834. 10.1084/jem.20101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graziano F, Desdouits M, Garzetti L, Podini P, Alfano M, et al. (2015) Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc Natl Acad Sci U S A 112: E3265–3273. 10.1073/pnas.1500656112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman DJ, Kunzli BM, YI AR, Sevigny J, Berberat PO, et al. (2009) From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 106: 16788–16793. 10.1073/pnas.0902869106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takenaka MC, Robson S, Quintana FJ (2016) Regulation of the T Cell Response by CD39. Trends Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai SH, Takeda K (2016) Regulation of allergic inflammation by the ectoenzyme E-NPP3 (CD203c) on basophils and mast cells. Semin Immunopathol. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow-derived mast cells were transferred into KitW-sh/W-sh mice. At day 2 and day 6 after the reconstitution, CD3- CD4- CD8- B220- cells were gated and frequencies of mast cells in the small intestine were analyzed for expression of c-kit+ FcεRI+ by flow cytometory.
(TIFF)
(A) Frequency and number of CD4+ T cells and B220+ B cells in the PPs and SILP of wild-type (n = 6) and Enpp3-/- (n = 6) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of CD4+ or B220+ cells are shown (right). NS: not significant. (B) Frequency and number of IL-17-producing CD4+ T cells in the small intestine of wild-type (n = 5) and Enpp3-/- (n = 5) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of IL-17+ CD4+ cells are shown (right). *p < 0.05, NS: not significant. (C) Frequency and numbers of CD4+ ICOS+ CXCR5+ follicular helper T (Tfh) cells in the PPs of wild-type (n = 5) and Enpp3-/- (n = 5) mice. Representative dot plots are shown (left) and the means ± SD of the percentages and total numbers of Tfh cells are shown (right). NS: not significant.
(TIFF)
(A, B) Frequency of CD45+ PDCA-1+ CD11cint pDCs and CD45+ PDCA-1- CD11c high cDCs in the PPs, SILP (A), BM, and SPL (B) of wild-type and Enpp3-/- mice. Representative dot plots are shown. Numbers in dot plots indicate the percentages of cells in the respective areas. (C) Frequency of PDCA-1+ CD11cint pDCs in the PPs and SILP from antibiotic-treated wild-type (n = 11) and Enpp3-/- (n = 12) mice or untreated wild-type (n = 10) and Enpp3-/- (n = 10) mice. Representative dot plots are shown. Numbers in dot plots indicate the percentages of cells in the respective areas.
(TIFF)
(A) Surface expression of Siglec H, CCR9 and CD45RA on CD45+ PDCA-1+ CD11cmed pDCs from SILP analyzed by flow cytometry. (B) CD45+ PDCA-1+ CD11cmed pDCs were isolated from SILP of wild-type and Enpp3-/- mice with FACS Aria. pDCs were stimulated with CpG DNA (5 μM) for 4 h. Expression of Ifna1 and Il12b was analyzed by quantitative RT-PCR (n = 3). NS: not significant.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.