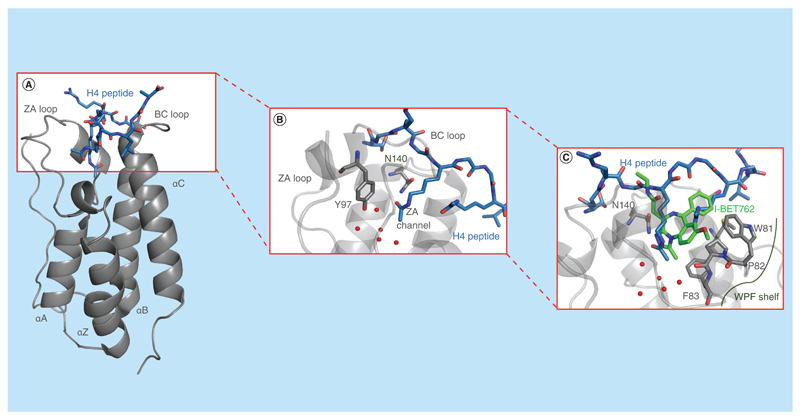
Figure 2. Structure and molecular recognition of BET bromodomains.
(A) X-ray structure of the di-acetylated H4 peptide (double acetylation at H4K5acK8ac, in blue) bound to the BET bromodomain BRD4-BD1 (in gray, PDB 3UVW). (B) Highlighted the conserved Y97, N140 and the ZA channel of BRD4-BD1(PDB 3UVW). (C) Superposition of the di-acetylated H4 peptide (in blue, PDB 3UVW) and the I-BET762 inhibitor (in green, PBD 3P5O) bound to BRD4-BD1 (in gray), highlighting the residues forming the WPF shelf.