Abstract
Background
It has been shown that birch pollen immunotherapy can induce IgG antibodies which enhance IgE binding to Bet v 1. We aimed to develop a serological assay to predict the development of antibodies which enhance IgE binding to Bet v 1 during immunotherapy.
Methods
In 18 patients treated by Bet v 1-fragment-specific immunotherapy, the effects of IgG antibodies specific for the fragments on the binding of IgE antibodies to Bet v 1 were measured by ELISA. Blocking and possible enhancing effects on IgE binding were compared with skin sensitivity to Bet v 1 after treatment.
Results
We found that fragment-specific IgG enhanced IgE binding to Bet v 1 in two patients who also showed an increase of skin sensitivity to Bet v 1.
Conclusion
Our results indicate that it may be possible to develop serological tests which predict the induction of unfavourable IgG antibodies enhancing the binding of IgE to Bet v 1 during immunotherapy.
Keywords: Bet v 1, birch pollen allergy, enhancing IgG antibodies, immunotherapy, recombinant allergen
Birch pollen allergy is a highly prevalent disease (1), which is due to IgE sensitization to the major allergen of birch, Bet v 1, a 17-kDa protein harbouring mainly conformational IgE epitopes (2). Birch pollen-specific immunotherapy (SIT) with birch pollen extract or purified recombinant Bet v 1 is a clinically effective treatment, and it has been shown that clinical improvement is associated with a reduction in birch pollen-induced immediate skin reactions and the development of Bet v 1-specific IgG antibodies (3). However, the molecular and immunological characterization of human Bet v 1-specific IgG antibodies has demonstrated that besides IgG antibodies, which inhibit allergic patients’ IgE binding to Bet v 1 and therefore can block Bet v 1-induced allergic reactions, two other types of Bet v 1-specific antibodies can develop in patients (4, 5). One type of these Bet v 1-specific IgG antibodies recognizes other epitopes than patients’ IgE, and hence, these antibodies do not inhibit IgE binding to Bet v 1. Another type of IgG antibodies was found to even enhance IgE binding to Bet v 1 and Bet v 1-induced immediate-type skin reactions (4, 5). The induction of the latter type of Bet v 1-specific IgG antibodies during SIT is therefore not desirable. Although it may be assumed that enhancing antibodies of the IgG isotype and potentially also of other isotypes may develop during immunotherapy against other allergen sources, to our knowledge, the presence of such undesirable antibodies has only been proven in birch pollen-allergic patients.
In this study, we investigated whether it is possible to develop an in vitro test that may predict the development of such nonbeneficial Bet v 1-specific IgG antibodies during injection immunotherapy.
Materials and methods
We used serum samples from 18 birch pollen-allergic patients obtained before and after SIT with recombinant Bet v 1 fragments (6). The presera of the patients were obtained before injection 1 of the treatment course. Bet v 1-specific IgG and IgE levels were measured by ImmunoCAP (Phadia, Uppsala, Sweden) (6).
In these serum samples, we determined changes (i.e. reduction or enhancement) in IgE binding to immune complexes consisting of Bet v 1 and IgG antibodies from rabbits that had been immunized with the same Bet v 1 fragments that had been injected into the patients (7). For this purpose, we performed an ELISA experiment with Bet v 1 on the solid phase and assessed to what extent the binding of IgE in patients’ sera (obtained before treatment) to the allergen was altered by pre-incubation of plate-bound Bet v 1 with fragment-specific rabbit IgG. The ELISA experiments are described in the online repository. ELISA results represent mean values of duplicate determinations with a deviation of <5%. The factor of blocking/enhancing of IgE binding by Bet v 1 fragment-specific rabbit IgG was calculated according to the following formula: Factor of change of IgE binding by rabbit IgG = absorbance fragment-specific IgG/absorbance control rabbit IgG. Thus, values >1 express an enhancement, whereas values <1 express an inhibition of IgE binding by fragment-specific rabbit IgG, respectively.
The results of the ELISA were compared with the induction of Bet v 1-specific IgG and the alterations of cutaneous and nasal sensitivity to Bet v 1 in the patients in the course of SIT (Fig. 1, Table 1 right column). Methods for skin prick testing and nasal provocation testing are described in the online repository.
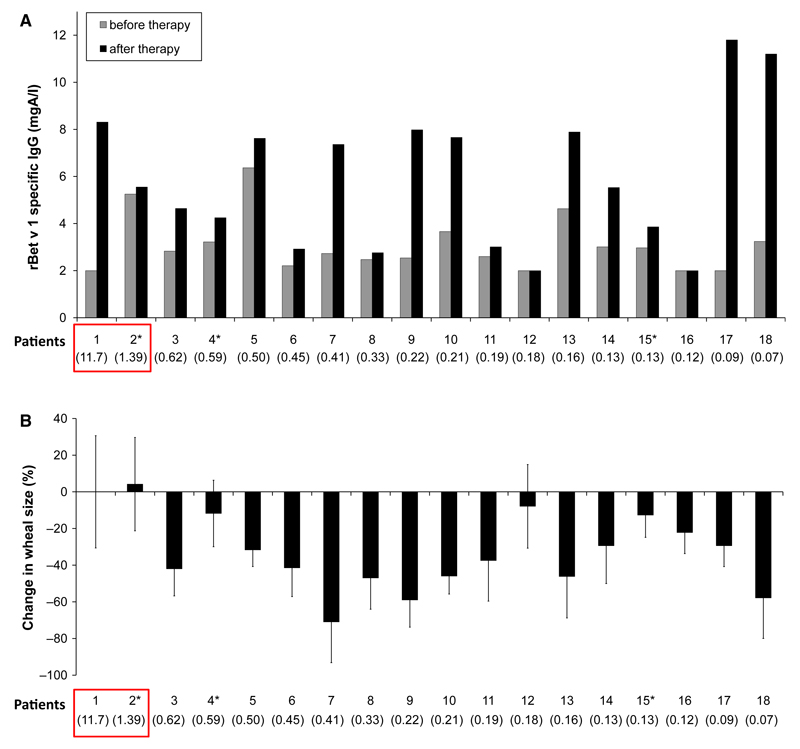
Figure 1.
(A) Bet v 1-specific IgG (mgA/l – milligram antigen per litre) before and after immunotherapy (y-axis) for each patient (after immunotherapy in May; patients 2*, 4* and 15* in October). (B) Change in immediate-type skin response after SIT in May (patient 2*, 4* and 15*: October) compared with values before immunotherapy. (A, B) The factor of (i.e. fold) change in IgE binding to Bet v 1 by fragment-specific IgG is shown for each of the patients (x-axes, in parentheses).
Table 1.
Patient data: demographic data, Bet v 1-specific IgE levels (kUA/L: kilo units antigen per litre), factors of (i.e. folds) change in IgE binding to Bet v 1 by fragment-specific IgG, numbers of injections and cumulative injected dose (CID) are displayed. In the right column, changes in nasal sensitivity to birch pollen extract as determined by nasal provocation tests before therapy and 1 year thereafter are shown: ’+’: improvement, ’−’: deterioration, ’=’: no change in nasal sensitivity to birch pollen extract. n.d.: not done
| Patient number | Sex | Age | Bet v 1-spec. IgE (kuA/L) | Factor of change of IgE binding by fragment-specific IgG | Immunizations |
Nasal tolerance | |
|---|---|---|---|---|---|---|---|
| Number | CID (µg) | ||||||
| 1 | m | 36 | 2.22 | 11.7 | 9 | 245.00 | = |
| 2 | f | 23 | 5.46 | 1.39 | 3 | 4.00 | − |
| 3 | m | 28 | 2.33 | 0.62 | 9 | 167.00 | − |
| 4 | f | 49 | 67.7 | 0.59 | 6 | 9.00 | n.d. |
| 5 | m | 33 | 10.8 | 0.50 | 9 | 245.00 | + |
| 6 | f | 45 | 11.6 | 0.45 | 8 | 165.00 | = |
| 7 | f | 23 | 13.3 | 0.41 | 8 | 165.00 | + |
| 8 | f | 38 | 25.7 | 0.33 | 7 | 85.00 | + |
| 9 | m | 51 | >100 | 0.22 | 7 | 85.00 | + |
| 10 | f | 45 | 24.7 | 0.21 | 9 | 103.00 | + |
| 11 | f | 48 | 26.2 | 0.19 | 9 | 185.00 | = |
| 12 | m | 31 | 7.35 | 0.18 | 3 | 5.00 | = |
| 13 | m | 35 | 58.1 | 0.16 | 9 | 205.00 | − |
| 14 | f | 58 | 41.1 | 0.13 | 9 | 88.00 | = |
| 15 | m | 34 | 8.4 | 0.13 | 7 | 28.00 | = |
| 16 | m | 33 | 39.70 | 0.12 | 5 | 25.00 | + |
| 17 | f | 25 | 24.4 | 0.09 | 9 | 245.00 | + |
| 18 | f | 41 | 56.4 | 0.07 | 9 | 197.00 | = |
Results
In Figures 1A and B, patients were ordered from right to left according to the extent of inhibition of IgE binding (maximum inhibition, right – no inhibition and enhancement, left). In 16 of the 18 patients, a blocking of IgE binding to Bet v 1 (factor 0.62 –0.07) by fragment-specific IgG was observed. Interestingly, in two patients, fragment-specific IgG enhanced the binding of IgE to Bet v 1 (Fig. 1, Table 1 and Table S1: Patient 1: factor 11.7; patient 2: factor 1.39).
We then assessed the levels of Bet v 1-specific IgG that was developed by the patients after SIT (Fig. 1). We found that 15 of the 18 patients showed increases in Bet v 1-specific IgG antibodies, and in two (i.e. patients 12 and 16), no relevant increase was detected. Next, we analysed the changes in immediate-type skin responses to Bet v 1 before and after the treatment. Two patients (i.e. patient 1 > patient 2) showed a development of Bet v 1-specific IgG during SIT, but showed no improvement in skin reactivity to Bet v 1. Furthermore, these two patients showed either no change or a deterioration of nasal sensitivity to birch pollen extract (Table 1, right column). Interestingly, for these two patients, we found that fragment-specific IgG enhanced IgE binding to Bet v 1 (Fig. 1). The development of IgG enhancing allergen-specific IgE in the patients may explain why they did not show a reduction in skin sensitivity after SIT and no improvement in nasal tolerance for birch pollen extract. In fact, patient 1 indeed failed to develop IgG antibodies against the major IgE-reactive area described by Gieras (2) (data not shown).
Discussion
Several research groups that develop allergy vaccines immunize animals with their vaccine candidates to study whether the vaccine can induce IgG antibodies upon immunization which block allergic patients’ IgE binding to the allergen (8). Such tests have been used for the evaluation of various recombinant allergen-based vaccines and in particular for vaccines that contain recombinant modified allergen derivatives before these derivatives underwent clinical testing (9).
For birch pollen allergy, it was observed that SIT may induce an unfavourable IgG antibody response, which enhances allergic patient’s IgE binding to Bet v 1 (4, 5). Therefore, we were interested whether it may be possible to use IgG antibodies induced in an experimental animal system with Bet v 1-based vaccines to identify patients whose IgE binding to Bet v 1 may be enhanced by Bet v 1-specific SIT. Our finding that exactly those patients whose IgE binding to Bet v 1 was enhanced by Bet v 1-specific IgG showed deterioration or no improvement in skin responses to Bet v 1, and no improvement in nasal tolerance of birch pollen extract after SIT indicates that it may indeed be possible to develop serological tests that may predict the development of unwanted IgG in the course of SIT.
Our results also indicate that not only the mere increase in allergen-specific IgG levels after SIT but also the quality (i.e. affinity, avidity, epitope specificity) of therapy-induced IgG is important for success of SIT (10). In conclusion, we provide evidence that it may be possible to develop tests that can predict the quality of allergen-specific IgG responses during SIT. The test system developed by us may depend on the animals used for immunization and the vaccine formulation, but outbred rabbits seem to be a quite good model system. Our results suggest that this system allows the prediction of the development of unfavourable IgG in the course of Bet v 1-specific SIT and may be useful to identify patients who will not benefit from Bet v 1-specific SIT. A similar approach could potentially also be developed to evaluate vaccines against other allergen sources. It may also be used to evaluate new forms of recombinant allergen-based allergy vaccines, which can focus IgG responses towards IgE-binding sites on allergens and may improve the efficacy of birch SIT in the future (11, 12).
Supporting Information
Additional Supporting Information may be found in the online version of this article
Acknowledgments
We thank Irene Steiner, MSc, Center of Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna, for her help regarding the statistical analysis of our data.
Funding
Supported by grants F4602, F4604 and F4613 of the Austrian Science Fund (FWF).
Footnotes
Conflict of interest
R.V. has received grant support from Phadia/Thermofisher, Uppsala, Sweden, and BIOMAY AG, Vienna, Austria, and serves as consultant for both companies. V.N. serves as a consultant for BIOMAY AG.
References
- 1.Swoboda I, Twaroch T, Valenta R. Tree pollen allergens. In: Kaliner MA, Lockey RF, editors. Allergens and allergen immunotherapy. 4th edn. New York: Informa Healthcare; 2008. pp. 87–105. [Google Scholar]
- 2.Gieras A, Cejka P, Blatt K, Focke-Tejkl M, Linhart B, Flicker S, et al. Mapping of conformational IgE epitopes with peptidespecific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J Immunol. 2011;186:5333–5344. doi: 10.4049/jimmunol.1000804. [DOI] [PubMed] [Google Scholar]
- 3.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Visco V, Dolecek C, Denepoux S, Le Mao J, Guret C, Rousset F, et al. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J Immunol. 1996;157:956–962. [PubMed] [Google Scholar]
- 5.Denéepoux S, Eibensteiner PB, Steinberger P, Vrtala S, Visco V, Weyer A, et al. Molecular characterization of human IgG monoclonal antibodies specific for the major birch pollen allergen Bet v 1. Anti-allergen IgG can enhance the anaphylactic reaction. FEBS Lett. 2000;465:39–46. doi: 10.1016/s0014-5793(99)01703-2. [DOI] [PubMed] [Google Scholar]
- 6.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D, et al. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J Immunol. 2000;165:6653–6659. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- 8.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 9.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 10.Shamji MH, Ljorring C, Francis JN, Calderon MA, Larche M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 11.Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji M, Layhadi J, et al. A non-allergenic birch pollen allergy vaccine consisting of hepatitis PreS-fused Bet v 1 peptides focuses blocking IgG towards IgE epitopes and shifts immune responses to a tolerogenic and Th1 phenotype. J Immunol. 2013;190:3068–3078. doi: 10.4049/jimmunol.1202441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Focke-Tejkl M, Valenta R. Safety of engineered allergen-specific immunotherapy vaccines. Curr Opin Allergy Clin Immunol. 2012;12:555–563. doi: 10.1097/ACI.0b013e328357ca53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.