Abstract
Background
The introduction of novel prognostic factors such as minimal residual disease (MRD) and genomic profiling in acute lymphocytic leukemia (ALL) has led to the reevaluation of the role of cytogenetics and other conventional factors in risk stratification.
Methods
We assessed the impact of baseline cytogenetics on the outcome of 428 adult patients with Philadelphia chromosome-negative ALL receiving frontline chemotherapy. Three hundred and thirty patients (77%) were treated with Hyper-CVAD-based regimens and 98 (23%) with augmented BFM.
Results
Median age was 40 years (range, 13-86). One hundred eighty-six patients (43%) had diploid cytogenetics, 32 (7%) had complex cytogenetics (defined as ≥5 chromosomal abnormalities), 27 (6%) had low hypodiploidy/near triploidy (Ho-Tr), 25 (6%) had high hyperdiploidy (HeH), and 24 (6%) had MLL rearrangement. Patients with MLL rearrangement, Ho-Tr and complex karyotype had significantly worse relapse-free survival (RFS) and overall survival (OS) when compared to the diploid group. By multivariate analysis including all baseline characteristics and MRD status, Ho-Tr and complex karyotype were independent predictive factors for worse RFS and OS. Furthermore, survival among all cytogenetic groups was similar regardless of the treatment received.
Conclusions
Complex karyotype and Ho-Tr are adverse prognostic factors in adults with ALL independent of MRD status. These findings suggest that pretreatment cytogenetics remain a valuable prognostic tool in this population.
Keywords: Acute lymphoblastic leukemia, cytogenetics, complex, hypodiploidy, prognosis, minimal residual disease
Graphical Abstract
Condensed Abstract: In adult patient with ALL, low hypodiploidy/near triploidy and complex karyotype are associated with worse survival independent of MRD response. Pretreatment cytogenetics should still be used for risk stratification of patients with ALL.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is a genetically and clinically heterogeneous disease.1 Despite the high response rate of ALL with standard multiagent chemotherapy, the majority of patients still relapse.2,3 The treatment arsenal for ALL has recently expanded with the development of novel monoclonal antibodies such as inotuzumab ozogamicin and blinatumomab which are able to achieve profound responses even in patients with heavily pretreated relapsed or refractory disease,4,5 and there is significant interest in moving these agents to the frontline setting. Early identification of patients at high risk of relapse is important for the development of comprehensive strategies that use all available treatment modalities in order to prevent relapse and improve outcomes in these patients.
The success of multiagent combination chemotherapy regimens for the treatment of pediatric and adult ALL is likely due to their ability to overcome the heterogeneity of this disease. ALL is commonly associated with various structural chromosomal abnormalities and changes in the total number of chromosomes, or ploidy, which represent its underlying genetic heterogeneity. There have been conflicting reports on the prognostic impact of these chromosomal changes in adult ALL. While Moorman and colleagues reported that complex karyotype, low hypodiploidy (Ho) and near triploidy (Tr) are independent adverse prognostic factors in adult ALL6,7, recent reports on patients treated with pediatric-inspired regimens have failed to confirm these findings.8,9 Conclusive answers to the clinical impact of ploidy in adult ALL have been difficult to obtain given the relatively low frequency of each of these cytogenetic subgroups. Furthermore, in recent years, minimal residual disease (MRD) assessment has emerged as a strong predictor for relapse and survival in ALL.10,11
To this end, we sought to determine the prognostic impact of complex karyotype and ploidy cytogenetic subgroups in adult patients with Philadelphia chromosome-negative (Ph-) ALL.
METHODS
Patients
We conducted a retrospective analysis that included patients enrolled on clinical trials investigating frontline chemotherapy for ALL. (NCT00671658, NCT00866749, NCT00501826, NCT01363128, NCT01371630). Between May 2000 and March 2015, 428 adult patients with previously untreated Ph- ALL received frontline chemotherapy at our institution with either hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (Hyper-CVAD) or augmented Berlin-Frankfurt-Munster (augmented BFM)-based chemotherapy and had an adequate pretreatment cytogenetic assessment. All patients signed an informed consent form for clinical trial participation, and all trials were approved by the institutional review board of The University of Texas MD Anderson Cancer Center (MDACC).
Treatments
Three hundred and thirty patients (77%) were treated with Hyper-CVAD-based regimens and 98 (23%) with augmented BFM. Augmented BFM was only administered in patients < 40 years of age. The details of these regimens are published elsewhere.12,13 All patients with CD20-positive ALL treated with Hyper-CVAD-based regimens received anti-CD20 monoclonal antibodies.
Response and Outcome Definitions
Complete remission (CR) was defined as the presence of < 5% blasts in the bone marrow (BM) with > 1 × 109/L neutrophils and > 100 × 109/L platelets in the peripheral blood with no evidence of extramedullary disease. CR with inadequate platelet response (CRp) was defined as meeting criteria for CR but with a platelet count ≤ 100 × 109/L. Relapse was defined by recurrence of ≥ 5% blasts in a BM aspirate or by the presence of extramedullary disease. Overall survival (OS) was calculated from the time of treatment initiation to death from any cause. Relapse-free survival (RFS) was measured from the date of CR/CRp to relapse or death from any cause.
Cytogenetic analysis
BM specimens for cytogenetic assessment were obtained prior to initiation of treatment in all patients. Cytogenetic studies were performed using standard G-banding technique at the MDACC Cytogenetic Laboratory. Karyotypes were interpreted using the International System for Cytogenetic Nomenclature criteria by expert pathologists at our institution.14
Designation as diploid karyotype required a complete analysis of at least 10 metaphases with good-quality banding. Patients were classified according to the MRC UKALLXII/ECOG E2993 karyotypic categories.6 Ho, defined by the presence of 30-39 chromosomes, and Tr, defined as 60-78 chromosomes, were combined for purposes of analysis as previously described.15 No patients with near haploidy (< 30 chromosomes) were identified in this cohort. High hyperdiploidy (HeH) was defined by the presence of 51-65 chromosomes, and tetraploidy (Tt) as ≥ 80 chromosomes. The definition of complex karyotype has been reported in the literature as either ≥ 3 or ≥ 5 chromosomal abnormalities.6,16 Thus, analysis of both of these groups was performed compared to the diploid group in order to determine the most prognostic definition of a complex karyotype; those abnormalities included any abnormality except for established, recurrent translocations such as t(4;11)(q21;q23), other MLL/11q23 translocations or t(1;19)(q21; p13.3) for example. All other non-diploid karyotypes were further categorized according to the structural changes which included chromosomal translocations, gains and losses. The categorization of patients into all the mentioned subgroups was performed by two independent observers including an expert hematopathologist.
MRD Assessment
Multiparameter flow cytometry (MFC) was used as previously described to assess for MRD. 10 MRD assessment was performed at the time of CR. Initially a 15-marker, 4-color panel was used; later, a 6-color panel was used. An aberrant population was defined as a cluster of at least 20 cells, and MRD positive value was assigned when there was expression of 2 or more aberrant antigens. The sensitivity of this MRD assay was 0.01%.
Statistical analysis
The relationships between cytogenetic subgroups and categorical variables were analyzed using the Fisher exact test, and the Kruskal-Wallis test was used to compare continuous variables. RFS and OS were calculated using Kaplan-Meier estimates, and survival estimates were compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model for the risk of relapse or death. Cytogenetic groups, T-cell lineage status, sex, performance status, chemotherapy regimen and MRD status at CR were included as categorical variables, whereas age, blood tests including white blood cells count (WBC) and blast percentage were treated as continuous variables. Models were fitted using stepwise forward selection with variables added to the model if P ≤ 0.05 in the univariate analysis. Computations were performed using SAS (version 9.4), TIBCO Spotfire S+ (version 8.2) and Graphpad Prism (version 6.07) statistical programs.
RESULTS
Description of the chromosomal abnormalities
Among the 428 evaluable patients, 186 patients (43%) had a diploid karyotype. Chromosomal abnormalities forming a complex karyotype did not include known recurrent translocations in ALL as previously described in the literature.6 Twenty four patients (6%) had 3 or 4 chromosomal abnormalities; 32 (7%) had 5 or more chromosomal abnormalities; Twenty seven patients (6%) had low hypodiploidy or near triploidy (Ho-Tr); 24 (6%) had HeH; and only 6 patients (1%) had Tt. Twenty-four patients (6%) had MLL rearrangement (MLL). Forty-nine patients (12%) had previously described recurrent chromosomal abnormalities, most of them with concurrent multiple abnormalities (Figure 1). Other chromosomal abnormalities, not previously described as recurrent were present in 56 cases (13%).
Figure 1.
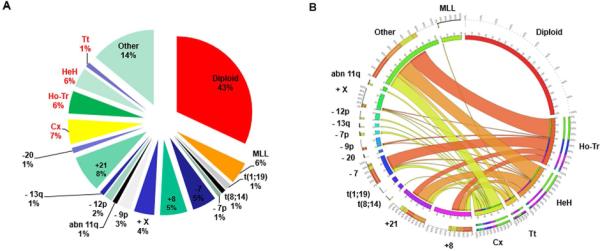
Cytogenetic architecture of adult Ph- ALL. (A) Relative frequency of the most common cytogenetics subgroups. Some patients had more than one of these abnormalities. ( B) Cytogenetic complexity of adult ALL. A circos diagram depicts the co-occurrence of the cytogenetic abnormalities. Only common chromosomal abnormalities are shown. Cx: complex; Ho-Tr: low hypodiploidy/near triploidy; HeH: high hyperdiploidy; Tt: tetraploidy.
Chromosomes were differentially involved among various ploidy groups and among patients with complex karyotype. Cytogenetic alterations in chromosomes 3, 7, 13, and 16 were most commonly present in the Ho-Tr in 82%, 75%, 72% and 72% respectively. In the HeH, the commonly altered chromosomes were chromosome 21 in 88%, chromosome 14 in 80%, and chromosome 4 in 68% of patients. Chromosomes 8, 5, and 7 were most commonly altered in the complex karyotype group with 46%, 46%, and 43% of patients, respectively. The median number of metaphases involved in patients with Ho-Tr, HeH, and complex karyotype was 8, 11 and 11 of 20 metaphases, respectively.
Clinical and hematologic presentation
Baseline characteristics are summarized in Table 1. The median age of the cohort was 40 years (range, 13-86). Patients with Ho-Tr were significantly older than those with diploid karyotype with a median age of 62 years (range, 18-78) vs 39 years (range, 15-84), respectively (P < 0.001). The Ho-Tr group also had a lower platelet count with a median count of 25 × 109/L compared to 45 × 109/L in the diploid group (P < 0.001). All patients in the Ho-Tr, HeH and MLL subgroups exclusively had a B-cell phenotype. Patients with a Tt karyotype were older than diploid patients (P < 0.001). Compared to diploid patients, those with MLL had a higher WBC and bone marrow blast percentage (P = 0.01).
Table 1.
Baseline characteristics, treatments and responses
| Characteristic | All (n=428) | Dp (n=186) | Ho-Tr (n=27) | HeH (n=24) | Cx (n=32) | MLL (n=24) | P |
|---|---|---|---|---|---|---|---|
| Age, years | 40 (13-86) | 39 (15-84) | 62 (18-78) | 40 (19-78) | 38 (19-72) | 42 (22-76) | 0.01 |
| Female n (%) | 186 (44) | 66 (35) | 14 (52) | 11 (45) | 11 (34) | 17 (70) | 0.02 |
| Male n (%) | 242 (56) | 120 (65) | 13 (48) | 13 (55) | 21(66) | 7 (30) | 0.02 |
| WBC, 109/L | 4.0 (0.4-602) | 3.9 (0.4-216) | 2.5 (0.7-86) | 2.5 (0.6-22) | 3.9 (0.5-87) | 9 (1-316) | 0.01 |
| Hg, g/L | 9.3 (3.5-16) | 9.3 (3.5-16) | 9.0 (4-11) | 9.2 (6.1-14) | 9.4 (5-14) | 9 (3.6-11.1) | 0.53 |
| Platelets, 109/L | 45 (0-626) | 65 (1-626) | 25 (7-233) | 46 (8-171) | 36 (0-361) | 33 (7-362) | 0.01 |
| Creatinine, mg/dL | 0.83 (0.3-4.0) | 0.9 (0.3-4.0) | 0.7 (0.4-2.3) | 0.9 (0.5-1.2) | 0.8 (0.5-2) | 0.8 (0.5-1.4) | 0.21 |
| Bilirubin, mg/dL | 0.5 (0.1-11) | 0.5 (0.1-8) | 0.65 (0.2-1.9) | 0.45 (0.2-1.3) | 0.6 (0.1-5) | 0.4 (0.2-1.7) | 0.25 |
| Albumin, mg/dL | 3.5 (1.9-5.2) | 3.6 (2.2-5.0) | 3.2 (2.5-4.4) | 3.4 (2.4-5.2) | 3.4 (1.9-4.1) | 3.5 (2.1-4.5) | 0.17 |
| Blasts in BM (%) | 84 (0-100) | 79 (0-99) | 80 (20-96) | 86 (26-99) | 88 (29-98) | 90 (28-96) | 0.01 |
| B-ALL, n (%) | 358 (84) | 150 (78) | 27 (100) | 24 (100) | 23 (72) | 24 (100) | 0.01 |
| T-ALL, n (%) | 70 (16) | 41 (22) | 0 (0) | 0 (0) | 9 (28.2) | 0 (0) | 0.01 |
| ECOG PS ≥ 2, n (%) | 56 (13) | 18 (9) | 8 (29) | 3 (12) | 3 (9) | 2 (8) | 0.12 |
| Treatment-related characteristics, n (%) | 0.08 | ||||||
| Hyper-CVAD | 330 (77) | 146 (78) | 25 (92) | 21 (87) | 23 (72) | 19 (79) | |
| Augmented BFM | 98 (23) | 40 (22) | 2 (8) | 3 (13) | 9 (28) | 5 (21) | |
| Morphologic response, n (%) | 0.57 | ||||||
| CR | 389 (91) | 174 (92) | 23 (86) | 22 (92) | 27 (85) | 21 (88) | |
| CRp | 11 (2) | 3 (2) | 2 (7) | 0 (0) | 2 (6) | 0 (0) | |
| PR | 3 (1) | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| NR | 12 (3) | 3 (2) | 0 (0) | 2 (8) | 2 (6) | 0 (0) | |
| Early death | 13 (3) | 3 (2) | 2 (7) | 0 (0) | 1 (3) | 3 (12) | |
| MRD at CR, n (%) | 0.28 | ||||||
| Positive | 96 (34) | 44 (37) | 3 (19) | 3 (16) | 12 (50) | 4 (29) | |
| Negative | 178 (62) | 70 (60) | 13 (81) | 13 (68) | 11 (46) | 9 (64) | |
| Indeterminate | 11 (4) | 4 (3) | 0 | 3 (16) | 1 (4) | 1 (7) | |
Continuous variables in the table are presented as median values and ranges. Abbreviations: y, years; Ho-Tr, Low hypodiploidy/near-triploidy HeH, high hyperdiploidy Cx, complex; MRD, ECOG PS, Eastern Cooperative Group performance status; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone; augmented BFM, augmented Berlin-Frankfurt-Munster. CR, complete response; CRp complete response with incomplete platelet recovery; PR, partial response; NR, no response. P is when all subgroups are compared.
Treatment Response
Overall, 389 patients (90%) achieved CR and 11 (2.5%) achieved CRp; 11 (2.5%) had resistant disease, and 8 (2%) died prior to first response assessment (Table 1). Patients in the different cytogenetic subgroups had similar responses. Among the 11 patients with resistant disease, 3 patients had diploid karyotype, 2 patients had HeH, 2 patients had a complex karyotype, and 4 had other chromosomal abnormalities.
MRD assessment by MFC was performed in 285 patients (73% of patients with CR/CRp). One hundred and seventy-eight patients (62%) were MRD negative at CR, 96 (34%) were MRD positive, and 11 (4%) had an indeterminate MRD assessment (Table 1). There was no difference in the rates of MRD negativity among the cytogenetic subgroups analyzed (P = 0.28).
Survival by Cytogenetics and MRD Assessment
With a median follow-up time for survivors of 58 months, the 5-year RFS and OS rates for the entire cohort were 36% and 47%, respectively. The OS of patients with 3 or 4 chromosomal abnormalities was similar to patients with diploid karyotype with 5-year OS rates of 53% (95% CI, 45%-62%) and 51 % (95% CI, 27%-63%), respectively (P = 0.51). In contrast, the 5-year OS rate of patients with 5 or more chromosomal abnormalities was 28 % (95% CI, 12%-46%), which was significantly worse than those with diploid karyotype (P < 0.001) (Figure 2). Thus, for purposes of further analyses, complex karyotype was defined as the presence of 5 or more chromosomal abnormalities. Furthermore, patients with other chromosomal abnormalities, present as 1 or 2 abnormalities, and not previously described as recurrent had a similar outcome as to those with diploid cytogenetic (P = 0.55).
Figure 2.
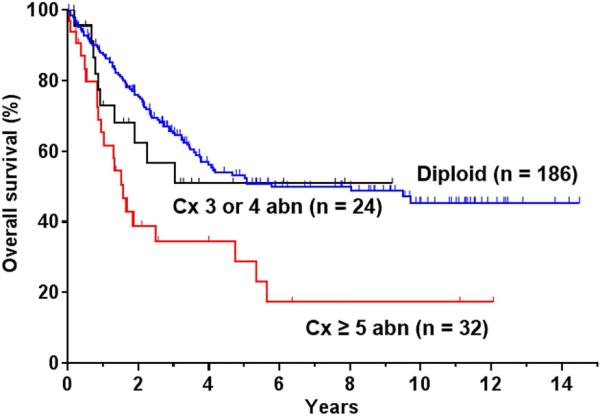
Overall survival of adult Ph- ALL patients with multiple chromosomal abnormalities defining a complex karyotype. Cx: complex; abn: abnormalities.
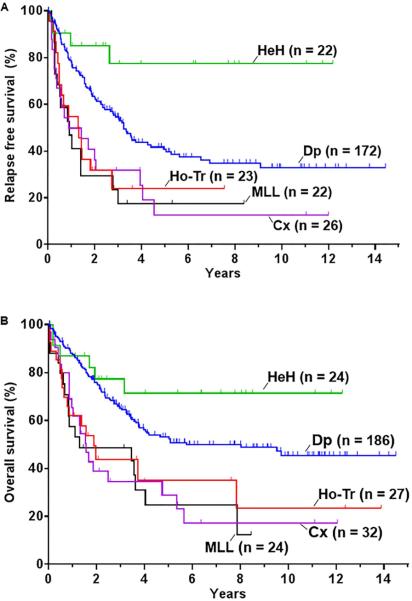
Patient with Ho-Tr, complex, or MLL had a worse RFS and OS when compared to the diploid group (Table 2; Figure 3). The 5-year RFS rates for the Ho-Tr group, complex, and patients with MLL translocation were 24%, 12%, and 18% respectively (Figure 3A). Similarly, the 5-year OS rates were 35%, 29%, and 25% respectively (Figure 3B). Patients with HeH had better outcome with respective 5-year RFS and OS rates of 77% and 71% (the difference in OS did not reach statistical significance) (Table 2; Figure 3). Thus patients with MLL rearrangement, Ho-Tr or complex karyotype were found to have poor risk disease. Subsequently, the survival impact of poor risk cytogenetics was assessed according to the chemotherapy regimen received. Patients with poor risk cytogenetics, treated with either Hyper-CVAD or augmented BFM had a similarly worse survival when compared to the diploid group with a median survival of 1.5 years and 1.6 years respectively (log rank P = 0.8).
Table 2.
Relapse free and overall survival among the cytogenetic subgroups of adult patients with Ph- ALL
| RFS (95% CI) |
OS (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Median (years) | % 5 years | P | Median (years) | % 5 years | P | |
| Dp (n=186) | 4.1 (3.2-9.4) | 41 (33-50) | - | 5.8 (3.8-NR) | 53 (46-62) | - |
| Ho-Tr (n=27) | 1.5 (0.9-NR) | 24 (10-55) | 0.003 | 1.9 (0.8-NR) | 35 (19-66) | 0.003 |
| HeH (n=24) | NR | 77 (60-100) | 0.014 | NR | 71 (54-94) | 0.151 |
| Cx (n=32) | 1.2 (0.5-4.0) | 12 (4-42) | 0.001 | 1.6 (0.9-5.4) | 29 (15-54) | 0.001 |
| MLL (n=24) | 1.2 (0.5-NR) | 18 (6-48) | 0.001 | 3.5 (0.8-NR) | 25 (11-55) | 0.001 |
Abbreviations: Dp, diploid; Ho-Tr, Low hpodiploidy/near-triploidy; HeH, high hyperdiploidy; Cx, complex; NR, not reached. Median survivals and survival rates were compared to the diploid group.
Figure 3.
survival of adult Ph- ALL patients according to the cytogenetic abnormalities. (A) Relapse free survival and (B) overall survival. Dp: diploid; Cx: complex; Ho-Tr: low hypodiploidy/near triploidy; HeH: high hyperdiploidy.
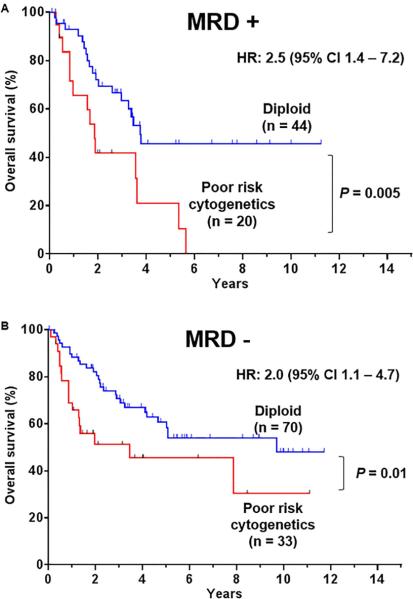
We subsequently assessed the impact of MRD status on the outcome of patients among the different cytogenetic subgroups. The median OS of patients with positive MRD and poor risk cytogenetics was 1.8 years versus 3.7 years for patients with positive MRD and diploid cytogenetics with a HR of 2.5 (95% CI, 1.4-7.2) (P = 0.005) (Figure 4A). The prognostic impact of the poor risk cytogenetics was still significant in patients who achieved MRD negativity (Figure 4B). The median OS for patients who achieved MRD negativity but had poor risk cytogenetics was 3.4 years versus 9.7 years for patients with MRD negativity and diploid cytogenetics with a HR of 2.0 (95% CI, 1.5-5.1)(P = 0.01). Thus, patients with poor risk cytogenetics and positive MRD at CR had the worse survival.
Figure 4.
Overall survival of adult Ph- ALL patients according to cytogenetics and MRD response at complete response. (A) Overall survival of adult Ph- ALL patients with MRD + response. (B) Overall survival of adult Ph- ALL patients with MRD – response. Poor risk cytogenetics includes MLL rearrangement, complex karyotype and low hypodiploidy/near triploidy.
A total of 30 patients (7%) underwent allogeneic stem cell transplant (ASCT) in first CR; fifteen (50%) of them had Ho-Tr, MLL or complex cytogenetic. Eighteen patients relapsed and 16 died. At the last follow-up, 14 patients (47%) remained alive; of whom 4 had poor risk cytogenetics.
Multivariate Analysis for Survival
The prognostic impact of the cytogenetic subgroups was evaluated in a multivariate analysis for RFS and OS (Table 3). By multivariate analysis, older age, higher WBC count, thrombocytopenia, Ho-Tr, complex cytogenetics and positive MRD at CR were independent predictive factors of poor RFS. Older age, higher WBC count, Ho-Tr, complex cytogenetics and positive MRD at CR were independent predictive factors of poor OS as well. The risk of death for patients with a complex karyotype was significantly higher than those with a diploid karyotype, independent of all the other factors analyzed, including MRD status, with a HR of 2.42 (95% CI, 1.26-4.66) (P = 0.01). Similarly, the risk of death for patients with Ho-Tr was higher than for diploid patients with a HR of 3.15 (95% CI 1.40-7.09) (P = 0.01). Despite worse survival by univariate analysis, MLL rearrangement was not independently predictive of either RFS or OS.
Table 3.
Multivariate analysis for the risk of relapse or death among patient with adult Ph- ALL.
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Risk of relapse or death | ||
| Age | 1.01 (1.01-1.02) | 0.03 |
| WBC | 1.24 (1.04-1.38) | 0.01 |
| Platelet count | 1.20 (1.04-1.38) | 0.01 |
| Performance status ≥ 2 | 1.81 (1.12-2.92) | 0.02 |
| Ho-Tr | 2.65 (1.28-5.52) | 0.01 |
| Complex karyotype | 2.58 (1.39-4.80) | < 0.01 |
| MRD positive | 1.82 (1.24-2.68) | < 0.01 |
| Risk of death | ||
| Age | 1.02 (1.01-1.03) | < 0.01 |
| WBC | 1.17 (1.01-1.36) | 0.04 |
| Performance status ≥ 2 | 1.72 (1.03-2.86) | 0.04 |
| Ho-Tr | 3.15 (1.40-7.09) | 0.01 |
| Complex karyotype | 2.42 (1.26-4.66) | 0.01 |
| MRD positive | 1.93 (1.27-2.92) | < 0.01 |
Ho-Tr, Low hpodiploidy/near-triploidy. WBC and platelet count were analyzed as log (WBC) and log (platelet). Decreased performance status was defined as ECOG PS ≥ 2. All cytogenetic subgroups were compared to the diploid subgroup.
DISCUSSION
In this study, we evaluated the impact of cytogenetic abnormalities in adult patients with Ph-negative ALL. We found that cytogenetic analysis adds independent prognostic information. A multivariate analysis confirmed the independent prognostic impact of complex karyotype and Ho-Tr even when MRD status was included in the analysis. In contrast, HeH was associated with an improved RFS and a trend towards an improved OS, as has been described in pediatric ALL.17 As previously described, patients with MLL rearrangement had a higher WBC, perhaps explaining why MLL was not identified as an independent factor in the multivariate analysis.6 Our findings further support the definition of complex karyotype in ALL as ≥ 5 chromosomal abnormalities. Reports have previously described complex karyotype as either ≥ 316 or ≥ 5 abnormalities6. We found that the clinical outcome of those two groups were drastically different. Survival of patients with ≥ 3 abnormalities was similar to that of patients with diploid karyotype contrasting with a significantly worse survival for patients with ≥ 5 abnormalities. Furthermore, the adverse outcome of poor risk cytogenetics defined as presence of complex karyotype, Ho-Tr or MLL rearrangement was similar in both the Hyper-CVAD and augmented BFM cohorts.
There have been previous studies attempting to determine the impact of cytogenetics with some conflicting results, the largest being the MRC UKALLXII/ECOG E2993 study 6,7,9,18. Moorman at al. identified in that study t(8;14)(q24.1;q32), Ho-Tr and complex karyotype as cytogenetic factors independently predictive of decreased RFS and OS. The independent prognostic value of cytogenetics was also confirmed in a study by the SWOG group.18 However, with the emergence of MRD as a strong prognostic marker and the identification of molecular markers such IKZF1 deletion and NOTCH mutation, Beldjord et al. from the GRAALL group reassessed conventional risk factors including cytogenetics.9 The GRAALL study confirmed the strong predictive value of MRD and found that MLL rearrangement is the only cytogenetic abnormality that carried prognostic significance. The differences between our findings and the French findings may be related to several factors. Patients in our cohort were older with a median age of 40 years (range, 13-86) vs 31 years (range, 15-60) in the GRAALL cohort. The age difference could possibly explain the higher incidence of Ho-Tr (11/423 in GRAALL cohort vs 27/428 in MDACC cohort) as Ho-Tr is more common in older patients.7 Other important factors are the inclusion of molecular data in the GRAALL study and the different methods by which MRD was assessed that could have led to different results (polymerase chain reaction versus MFC). Finally, only 7% of our patients received ASCT in CR1 compared to 37% in the French series. As such, those factors could have led to the different conclusions.
Numerous studies have underscored the importance of MRD as a biomarker, which has led to the reevaluation of historically prognostic pretreatment characteristics in ALL.10,11,19,20 There is no consensus on the optimal method for measuring MRD in ALL (e.g. MFC, polymerase chain reaction or next generation sequencing).21-23 Given the lack of standardization for MRD detection methods, it is difficult to compare MRD assessment across different laboratories and studies. Furthermore, it is difficult sometimes to have reliable and reproducible MRD assessment by MFC given that some expertise is needed especially in poor resource settings. Cytogenetic analysis has been performed for years, and methods of measurement and interpretation have been standardized, thus offering an easier prognostication of ALL. Having both MRD status and cytogenetics would allow for better risk stratification. Our study suggests that even if patients achieve MRD negative status, their outcome is worse if they have poor risk cytogenetics as defined by MLL rearrangement, Ho-Tr or complex karyotype. In contrast, patients with diploid cytogenetics and negative MRD status had a better outcome. Interestingly, patients with MLL rearrangement, Ho-Tr, and complex karyotype had MRD responses similar to those with diploid cytogenetics. This may indicate the presence of residual clones with those abnormalities at levels below MRD detection, which are capable of leukemogenesis and thus relapse. In pediatric ALL, low-hypodiploidy (32-39 chromosomes) has been most commonly associated with mutations in TP53, whereas near-haploidy (24-31 chromosomes) has been associated with alterations in the tyrosine kinase and Ras signaling.24 It is possible that similar associations can be found in adult ALL, explaining the higher rate of treatment failure associated with some of the cytogenetic abnormalities. Ongoing molecular profiling in our cohort of patients may help us understand the mechanisms of resistance in patients with poor risk cytogenetics, thus potentially identifying new mutations amenable for targeting in the future.
Our study could not assess whether ASCT can improve the prognosis of patients with poor risk cytogenetics. This was related to the relatively low frequencies of these abnormalities and the relatively low rate of transplantation in first remission of only 7%. This relatively smaller number of patients who underwent ASCT in first remission prohibited a meaningful analysis of the interaction between cytogenetic abnormalities and ASCT. Larger studies might be needed to address this point.
In conclusion, the presence of complex karyotype (defined as ≥5 chromosomal abnormalities) or Ho-Tr in adult ALL is independently predictive of RFS and OS even when MRD information is available. Results from this study can be used for risk stratification of adult ALL and allow for the development of comprehensive strategies aimed at improving the outcomes of patients with a high risk of relapse. Further studies are needed to identify the mechanisms of the genomic instability in ALL and the genomic drivers associated with those cytogenetic groups which could affect prognosis.
Acknowledgments
Funding source: Supported by the MD Anderson Cancer Center Support Grant CA016672,P30 CA016672, Ronald DePinho
Footnotes
Disclosure of Conflicts of Interest: The authors report no conflicts of interest.
Authorship Contributions: G.C.I. designed the study, collected and analyzed the data, and wrote the manuscript; H.K. and E.J. designed the study, collected and analyzed the data, treated patients, and wrote the manuscript; W.Q. and K.S. performed the statistical analysis; C.C.Y assisted with pathologic interpretation; S.P. and N.J.S collected and analyzed the data; F.R., D.T., G.G.M., T.M.K., J.E.C, N.D., G.B., N.J., M.K., N.D., I.K., P.K., R.E.C and S.M.O treated patients. All authors reviewed and approved the manuscript.
REFERENCES
- 1.Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–61. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 2.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. British journal of haematology. 2012;157:463–71. doi: 10.1111/j.1365-2141.2012.09095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–50. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. The Lancet Oncology. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. The Lancet Oncology. 2012;13:403–11. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 6.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–97. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 7.Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115:206–14. doi: 10.1182/blood-2009-07-232124. [DOI] [PubMed] [Google Scholar]
- 8.Brandwein JM, Atenafu EG, Schuh AC, et al. Predictors of outcome in adults with BCR-ABL negative acute lymphoblastic leukemia treated with a pediatric-based regimen. Leukemia research. 2014;38:532–6. doi: 10.1016/j.leukres.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123:3739–49. doi: 10.1182/blood-2014-01-547695. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi F, Jorgensen JL, O'Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. British journal of haematology. 2016;172:392–400. doi: 10.1111/bjh.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortuza FY, Papaioannou M, Moreira IM, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:1094–104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 13.Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2222–30. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 14.ISCN Shaffer LGSM, Campbell LJ, editors. An International System for Human Cytogenetic Nomenclature. 2009 [Google Scholar]
- 15.Charrin C, Thomas X, Ffrench M, et al. A report from the LALA-94 and LALA-SA groups on hypodiploidy with 30 to 39 chromosomes and near-triploidy: 2 possible expressions of a sole entity conferring poor prognosis in adult acute lymphoblastic leukemia (ALL). Blood. 2004;104:2444–51. doi: 10.1182/blood-2003-04-1299. [DOI] [PubMed] [Google Scholar]
- 16.Jarosova M, Holzerova M, Mihal V, et al. Complex karyotypes in childhood acute lymphoblastic leukemia: cytogenetic and molecular cytogenetic study of 21 cases. Cancer genetics and cytogenetics. 2003;145:161–8. doi: 10.1016/s0165-4608(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 17.Williams DL, Tsiatis A, Brodeur GM, et al. Prognostic importance of chromosome number in 136 untreated children with acute lymphoblastic leukemia. Blood. 1982;60:864–71. [PubMed] [Google Scholar]
- 18.Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood. 2008;111:2563–72. doi: 10.1182/blood-2007-10-116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–6. [PubMed] [Google Scholar]
- 20.Cave H, van der Werff ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. The New England journal of medicine. 1998;339:591–8. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 21.Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2014;2014:244–9. doi: 10.1182/asheducation-2014.1.244. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen JJ, van der Velden VH, Bruggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125:3996–4009. doi: 10.1182/blood-2015-03-580027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassan R, Spinelli O. Minimal Residual Disease Monitoring in Adult ALL to Determine Therapy. Current hematologic malignancy reports. 2015;10:86–95. doi: 10.1007/s11899-015-0252-7. [DOI] [PubMed] [Google Scholar]
- 24.Holmfeldt L, Wei L, Diaz-Flores E, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nature genetics. 2013;45:242–52. doi: 10.1038/ng.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]