Abstract
Ribonucleotides are incorporated into genomes by DNA polymerases, they can be removed, and if not removed, they can have deleterious and beneficial consequences. Here, we describe an assay to quantify stable ribonucleotide incorporation by DNA polymerases in vitro, and an assay to probe for ribonucleotides in each of the two DNA strands of the yeast nuclear genome.
Keywords: Ribonucleotide incorporation, DNA replication, DNA polymerase, Ribonucleotide excision repair, Alkali-sensitive sites
1 Introduction
DNA is more stable than RNA because ribonucleotides contain a reactive 2′-hydroxyl on the ribose ring that greatly sensitizes the sugar–phosphate backbone to hydrolysis [1]. Moreover, ribonucleotides in DNA alter nucleic acid geometry and can potentially influence cellular DNA transactions and alter the information stored in DNA (reviewed in [2]). For these reasons, it is of interest to quantify the ability of DNA polymerases to incorporate ribonucleotides into DNA. Much of our current understanding of ribonucleotide incorporation by DNA polymerases comes from kinetic analysis of the two steps needed for stable incorporation, ribonucleotide insertion (e.g., see [3]) followed by extension from the resulting primer terminus. Kinetic approaches monitor each step individually, in reactions containing a single dNTP or rNTP, and usually at one or two template bases. The first part of this chapter describes an alternative method, which quantifies stable ribonucleotide incorporation by a DNA polymerase at multiple template bases in the same experiment and in a reaction containing all four dNTPs and all four rNTPs at physiologically relevant concentrations. Because ribonucleotides within DNA genomes can have consequences both deleterious and beneficial [2], it is also of interest to determine how many ribonucleotides are incorporated into DNA in a cell and into which strand, nascent leading or lagging, they are incorporated. In the second part of this chapter, we describe an approach to do this.
2 Materials
2.1 In Vitro Measurement of Stable Incorporation of Ribonucleotides into DNA
2.1.1 Purification of Unlabeled DNA Oligonucleotides Using Gel Electrophoresis
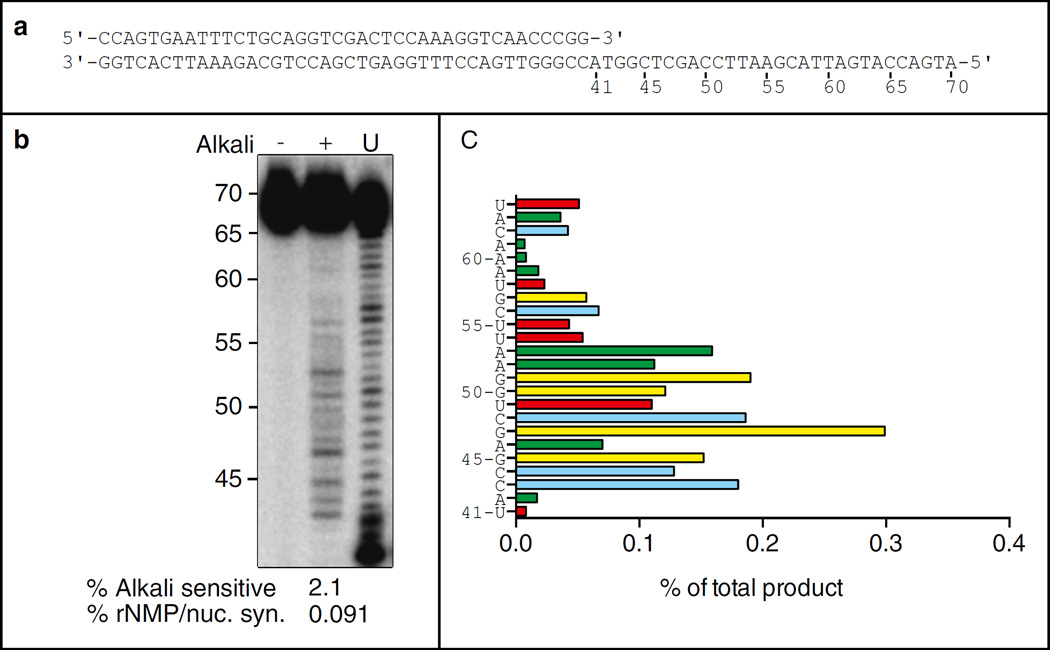
70-mer DNA template oligonucleotide (5′-ATGACCATGATTACGAATTCCAG CTCGGTACCGGGTTGACCTTTG GAGTCGACCTGCAGAAATTCACTGG) and 40-mer primer oligonucleotide (5′-CCAGTGAATTTCTGCAGGTC GACTCCAAAGGTCAACCCGG) [4] (Fig. 1a).
1× TBE: 89 mM Tris-borate, 2 mM EDTA, pH 8.3. Stored at room temperature.
8 % Acrylamide gel mix: 8 % acrylamide (from 40 % acrylamide/ bis-acrylamide 19:1 solution), 8 M urea in 1× TBE buffer. Can be prepared in advance and stored at room temperature and protected from sunlight.
Tetramethylethylendiamine (TEMED).
Ammonium persulfate: 10 % solution in water (APS) (see Note 1).
100 and 250 mL glass beakers.
50 mL pipettes.
Plastic Pasteur pipettes.
Formamide loading buffer: 95 % deionized formamide, 25 mM EDTA, 10 mg/mL bromophenol blue, 10 mg/mL xylene cyanol (see Note 1).
Sequencing gel electrophoresis apparatus, including glass plates.
Spacers and comb, 0.8 mm (see Note 2).
High-voltage power supply.
260 nm UV lamp.
Forceps.
Elution buffer: 0.1 % SDS, 0.5 M ammonium acetate, 10 mM magnesium acetate. Stored at room temperature.
3 M sodium acetate, pH 5.2. Stored at room temperature.
100 % ethanol.
70 % ethanol.
A column to remove gel pieces. We use low binding Durapore PVDF membrane (0.45 µm) (Millipore).
1× TE: 10 mM Tris–HCL, pH 8.0, 1 mM EDTA. Stored at room temperature.
UV-Vis NanoDrop Spectrophotometer.
Plastic wrap.
Clean razor blade.
Fig. 1.
(a) Sequence of primer-templates used for reactions in panel b. (b) Stable rNMP incorporation. Lane marked (U) depicts products generated by Pol ε prior to gel purification. (−) indicates KCl treatment and (+) indicates KOH treatment. The percentage of alkali-sensitive product and the percentage of rNMP incorporation per nucleotide synthesized is shown below the lane. (c) Percentage of rNMP incorporation by Pol ε at each of 24 template positions. The position and identity of each incorporated rNMP are displayed on the Y-axis
2.1.2 5′-End Labeling of Primer and Annealing
Gel-purified primer oligonucleotide from Subheading 2.1.1.
Gel-purified template oligonucleotide from Subheading 2.1.1.
γ-32P-ATP, 3000 Ci/mmol (see Note 3).
T4 polynucleotide kinase (PNK).
10× PNK buffer: 0.7 M Tris–HCl, pH 7.6, 0.1 M MgCl2, 50 mM dithiothreitol (DTT).
A column to remove unincorporated radioactive nucleotides. We use GE Healthcare G-25 spin column.
20× SSC: 3 M NaCl, 300 mM sodium citrate, pH 7.0.
2.1.3 Ribonucleotide Incorporation
5× reaction mixture: 200 mM Tris–HCl, pH 7.8, 1 mg/mL BSA, 5 mM DTT, 500 mM NaCl.
80 mM magnesium acetate (see Note 4).
10× “all dNTP” mixture: 160 µM dATP, 140 µM dCTP, 120 µM dGTP, 300 µM TTP (see Note 5).
10× “dNTP-NTP” mixture: 160 µM dATP, 140 µM dCTP, 120 µM dGTP, 300 µM TTP, 30 mM ATP, 5 mM CTP, 7 mM GTP, 17 mM UTP (see Note 5).
100 nM DNA Pol ε [5] or polymerase of interest.
Formamide loading buffer (see Subheading 2.1.1, item 9).
2.1.4 Isolating Full-Length DNA Products Using Gel Electrophoresis
Components as under Subheading 2.1.1, but with the following changes or additions:
Spacers and comb, 0.4 mm (see Note 2).
Cambrex Gel-Bond® PAG Film.
Office tape to align X-ray Film with Gel-Bond® PAG Film.
Hobby dye to align X-ray film with Gel-Bond® PAG γ-32P-ATP.
γ-32P-ATP (3000 Ci/mmol).
Geiger counter.
X-Ray film.
Full-face screen to be used during excision of radioactive full-length product from polyacrylamide gel.
Clean razor blade.
3MM CHR Whatman Chromatography paper.
Cotton swab.
1× TE buffer: 10 mM Tris–HCl, pH 8.0, 1 mM EDTA.
Gel dryer.
Phosphorimager and screen.
A column to remove gel pieces. We use low binding Durapore PVDF membrane (0.45 µm) (Millipore).
2.1.5 Counting, Alkaline Hydrolysis, and Gel Electrophoresis
Scintillation counter.
3.0 M KCl.
3.0 M KOH.
Formamide loading buffer (Subheading 2.1.1, item 9).
1× TBE: 89 mM Tris-borate, 2 mM EDTA, pH 8.3. Stored at room temperature.
8 % acrylamide gel mix: 8 % acrylamide (from 40 % acrylamide/ bis-acrylamide 19:1 solution), 8 M urea in 1× TBE buffer. Can be prepared in advance and stored at room temperature and protected from sunlight.
Tetramethylethylendiamine (TEMED).
Ammonium persulfate: 10 % solution in water (APS) (see Note 1).
100 and 250 mL glass beakers.
50 mL pipettes.
Plastic Pasteur pipettes.
Sequencing gel electrophoresis apparatus and glass plates for vertical polyacrylamide gels.
Spacers and comb, 0.8 mm (see Note 2).
High-voltage power supply.
3MM CHR Whatman Chromatography paper.
Phosphorimager and screen.
Software for quantification and analysis: ImageQuant and Microsoft Excel.
Plastic wrap.
2.2 Strand-Specific Probing for Alkali-Sensitive Sites in Yeast Genomic DNA
2.2.1 Isolation of Genomic DNA
Epicentre MasterPure Yeast DNA Purification Kit and materials and reagents listed in the kit instructions.
1× TE: 10 mM Tris-HCl, 1 mM EDTA, pH 8.0.
3 M sodium acetate, pH 5.2.
100 % ethanol.
70 % ethanol.
1 M KOH.
6× alkaline DNA loading buffer: 300 mM KOH, 6 mM EDTA, pH 8.0, 18 % Ficoll, 0.15 % bromocresol green, 0.25 % xylene cyanol FF. For 500 µL, combine 150 µL of 1 M KOH, 6 µL of 0.5 M EDTA, pH 8.0, 250 µL of 36 % Ficoll, 50 µL of 1.5 % bromocresol green, 50 µL of 2.5 % xylene cyanol FF (see Note 6).
5 µg/µL RNase A.
Qubit 2.0 flourometer with a dsDNA BR assay kit or similar.
2.2.2 Alkaline Agarose Gel Electrophoresis
Alkaline agarose gel: 1 % agarose, 50 mM NaOH, 1 mM EDTA, pH 8.0 (see Note 7).
DNA size marker.
0.5 µg/mL ethidium bromide.
1× alkaline electrophoresis buffer: 50 mM NaOH, 1 mM EDTA, pH 8.0. To make 2 L: use 10 mL of 10 N NaOH, 4 mL of 0.5 M EDTA, pH. 8.0, make up to 2 L with H2O.
Neutralization buffer I: 1 M Tris–HCl, 1.5 M NaCl. Take 315.2 g Trizma-HCl and 175.3 g NaCl, make up to 2 L with H2O. pH does not need to be adjusted.
UV transilluminator.
2.2.3 DNA Transfer to Membrane
Glass baking dish.
Amersham Hybond-N+ Nylon Membrane. Cut to the size of the alkaline agarose gel.
Three 100 mL glass beakers.
Two glass plates.
Paper towels (large stack cut to a size slightly larger than the agarose gel).
Thick blotting paper (Whatman 3MM). Two pieces cut to the size of the agarose gel and two longer pieces used to drape over the sides of the glass plate into the buffer reservoir.
Weight (≥400 g) to be placed on top of the transfer stack.
5 mL pipette.
Four strips of parafilm.
Alkaline transfer buffer: 0.4 N NaOH, 1 M NaCl.
Neutralization buffer II: 0.5 M Tris–HCl, pH 7.2, 1 M NaCl.
2.2.4 Preparation of Strand-Specific Single-Strand Probe
PCR product (see Subheading 3.2.4, step 1).
QIAquick PCR Purification Kit.
TaKaRa Ex Taq DNA Polymerase (5 U/µL) and 10× buffer.
α-32P-dCTP (see Note 3).
2.5 mM dNTPs (without dCTP).
GE Healthcare G-25 Spin Columns.
Primers (see Subheading 3.2.4, step 3).
2.2.5 Southern Hybridization
1 M sodium phosphate buffer, pH 7.2. Combine 280 mL of 1 M NaH2PO4 with 720 mL of 1 M Na2HPO4.
Hybridization buffer: 0.5 M sodium phosphate buffer, pH 7.2, 7 % SDS, 1 % BSA (see Note 8).
Phosphate-SDS Washing Solution I: 40 mM sodium phosphate buffer, pH 7.2, 5 % SDS, 0.5 % BSA, 1 mM EDTA, pH 8.0.
Phosphate-SDS Washing Solution II: 40 mM sodium phosphate buffer, pH 7.2, 1 % SDS, 1 mM EDTA, pH 8.0.
Hybridization oven and bottles.
Plastic sheet protector sleeve.
Tweezers.
Phosphorimager and screen.
2.2.6 Stripping the Blot
20× SSPE: 3 M NaCl, 0.2 M NaH2PO4, 0.02 M EDTA, pH 7.4. Weigh out 175.3 g NaCl, 27.6 g NaH2PO4 xH2O and 7.4 g EDTA and dissolve in 800 mL H2O. Adjust to pH 7.4 with NaOH and bring up the total volume to 1 L with H2O.
Blot Stripping Solution: 50 % formamide, 2× SSPE. 25 mL required per membrane. For 50 mL, add 25 mL of formamide, 5 mL of 2× SSPE and 20 mL of H2O.
Phosphate-SDS Washing Solution II (Subheading 2.2.5, item 4).
Geiger counter.
3 Methods
3.1 In Vitro Measurement of Stable Incorporation of Ribonucleotides into DNA
To measure the rate of stable incorporation of ribonucleotides by DNA polymerase of interest, an end-labeled primer is hybridized to the unlabeled template oligonucleotide. This substrate is then added to the polymerase reaction in the presence of physiological concentrations of dNTPs and NTPs. The resulting product is gel-purified and treated with alkali to introduce strand breaks at the sites of ribonucleotide incorporation. The untreated and the alkali-treated products are then compared on the gel, and the frequency of ribonucleotide incorporation is quantified [4, 6–9] (Fig. 1).
3.1.1 Purification of Unlabeled DNA Oligonucleotides Using Gel Electrophoresis
Mix 70 µL of TEMED and 140 mL of 8 % acrylamide gel mix in a 250 mL glass beaker. Add 740 µL of 10 % APS, mix and immediately pour mixture into gel cast with 0.8 mm spacers using a 50 mL pipette. Insert comb and keep the top of the gel uncovered until the remaining part of the gel mix in the beaker has polymerized (~10–60 min). Cover the gel with wet wipes, then cover the gel with plastic wrap and let it polymerize overnight.
Prerun the acrylamide gel for 30 min at 65 W. Rinse wells with buffer using a Pasteur pipette. Heat samples to 95 °C for 3 min and chill on ice for 3 min. Spin down the condensation for 10 s before loading. Load 5 µL of DNA (500 µM) + 5 µL of formamide loading buffer in each well (see Note 1).
Run the gel at 65 W for approximately 2 h until the bromophenol blue dye is located near the bottom of the gel.
Transfer the gel to plastic wrap.
Shadow with 260 nm UV lamp. Cut out using a razor blade the full-length product band from each lane, while covering the other lanes to avoid excessive UV exposure. Place the excised gel fragment in a 1.5 mL tube using forceps. Fragments from two replicate lanes can be placed in the same 1.5 mL tube.
Crush the gel slices in the tube using a P200 pipet tip (see Note 9) and a circular grinding motion.
Add 700 µL of Elution buffer per tube and rotate overnight at room temperature.
Remove solid matter using a 0.45 µm column with a Low binding Durapore PVDF membrane. Transfer the eluate to a 2 mL tube. Add 65 µL of 3 M sodium acetate, pH 5.2 and 1.3 mL of 100 % ethanol. Precipitate overnight at −20 °C.
Spin in a microfuge at maximum speed for 60 min at 4 °C and remove supernatant.
Wash with 500 µL of 70 % ethanol. Spin 30 min at 4 °C in a microfuge at maximum speed. Remove supernatant. Spin 10 s at maximum speed and remove the remaining supernatant.
Dry samples at 37 °C for 10 min to remove remaining ethanol.
Dissolve pellet in 20 µL of 1× TE buffer.
Measure the DNA concentration using a UV-Vis NanoDrop Spectrophotometer.
3.1.2 5′-End Labeling of Primer and Annealing
Label primer in a 25 µL reaction mixture containing 100 pmol of gel-purified primer, 2.5 µL of 10× PNK buffer, 5 µL fresh γ32P-ATP, 10 units of T4 PNK. Add the ATP last, and incubate for 20 min at 37 °C.
Inactivate the enzyme at 65 °C for 20 min.
Remove unincorporated γ32P-ATP using a G-25 spin column or similar: Spin 1 min at 720 × g in a centrifuge to remove buffer, load the reaction product to the center of the resin, spin for 2 min at 720 × g to elute oligo.
Mix the eluted primer with 150 pmol of template oligonucleotide and 1 µL of 20× SSC, add water to a final volume of 50 µL, vortex and spin tube.
Place in a beaker of water at 85 °C. Cool to room temperature. Spin tube and store at −20 °C.
3.1.3 Ribonucleotide Incorporation
- Prepare a 20 µL polymerase reaction mixture containing:
5× reaction mixture 4 µL 80 mM magnesium acetate (see Note 4) 2 µL 10× “all dNTP” mixture or 10× “dNTP-NTP” mixture 2 µL 2 µM DNA substrate (from Subheading 3.1.2, step 5) 2 µL H2O 8 µL Initiate reaction by adding 2 µL of DNA polymerase to a final concentration of 10 nM (see Note 10).
Incubate at 30 °C for 30 min.
Stop the reaction by adding 20 µL of formamide loading buffer.
3.1.4 Isolating Full-Length DNA Products Using Gel Electrophoresis
Mix 35 µL of TEMED and 70 mL of 8 % acrylamide gel mix in a 100 mL glass beaker. Add 370 µL of 10 % APS and immediately pour gel into gel cast with 0.4 mm spacers. Insert combs and keep the gel uncovered until the remaining part of the gel-mix in the beaker has polymerized after 10–60 min. Cover the gel with wipes and wet the wipes with water before covering the gel with plastic wrap and let polymerize overnight.
Prerun the acrylamide gel for 30 min at 65 W. Rinse the wells with buffer using a Pasteur pipette. Incubate DNA polymerase products at 95 °C for 3 min. Chill on ice for 3 min. Spin in a microfuge at max speed for 10 s and load five wells per variable with 6 µL of reaction mixture. Reserve a few microliters of the reaction mixture for scintillation counting in Subheading 3.1.5.
Run the gel at 65 W for approximately 90 min.
Mix 1 µL γ32P-ATP with 100 µL hobby dye in a 1.5 mL tube. Transfer the acrylamide gel to a Gel-Bond® PAG Film. Spot radioactive hobby dye on three corners of the gel using a cotton swab (see Note 11). Cover the gel with plastic wrap and expose to X-ray film for 1–2 h.
Develop X-ray film and align the developed X-ray film with the acrylamide gel using the spots from the hobby dye indicator. Tape X-ray film and Gel-Bond® PAG Film into place so that these are fixed and aligned. Use a full-face screen and cut out bands corresponding to the full-length product with a clean razor blade, and transfer the gel pieces to a 1.5 mL tube.
Purify the radioactively labeled DNA according to Subheading 3.1.1, steps 6–12. Resuspend the purified DNA products in 20 µL of 1× TE buffer.
Remove plastic wrap from acrylamide gel and replace with Whatman paper, dry gel on a gel-dyer for 1 h and expose to phosphorimager screen. Scan the screen to confirm excision of full-length products from the gel.
3.1.5 Counting, Alkaline Hydrolysis, and Gel Electrophoresis
Count in a scintillation counter 1 µL of unpurified (Subheading 3.1.4, step 2) and purified DNA (Subheading 3.1.4, step 6) products. The unpurified product is used as a ladder to identify the specific bands of the alkaline hydrolyzed products. Calculate the amount of each purified product needed to achieve the same number of counts in the scintillation counter. It is necessary to load equal amounts of unpurified product (ladder) and alkaline hydrolyzed products so that one of these does not become oversaturated during the scan of the gel. In addition, the initial substrate (Subheading 3.1.2, step 5) should be loaded at a tenfold less concentration to identify the beginning of the 40-mer primer: it has to be tenfold less to achieve a single band.
In a 1.5 mL tube, mix 1 µL 3 M KCl or 3 M KOH with purified product (equal number of scintillation counts for each product), add water to 10 µL. Incubate for 2 h at 55 °C.
Chill on ice for 5 min. Add 10 µL formamide loading buffer to the reaction and incubate at 95 °C for 3 min. Chill on ice for 3 min. Spin at max speed for 10 s.
Load 6 µL of each sample of DNA products and purified DNA products treated with KCl and KOH on an 8 % acrylamide 0.4 mm gel (see Subheading 3.1.4, steps 1–2) and load tenfold fewer counts for the initial substrate.
Run gel at 65 W until bromophenol blue reaches the bottom of the gel, transfer the gel to an old X-ray film or to plastic wrap and cover with 3 MM CHR Whatman Chromatography paper. Dry gel for 1 h on a gel-dryer.
Expose the gel for 1 h to a phosphorimager screen. After 1 h, place the screen in a phosphorimager scanner and scan gel. If the pixel counts are not sufficient, erase screen and incubate the dried gel with phosphorimager screen overnight or for several days.
Alkaline-hydrolyzed products migrate 1 nucleotide shorter compared to the unpurified products due to their 2′,3′ cyclic phosphate products (Fig. 1b). Count the pixels in KCl and KOH-treated lanes with ImageQuant software. Subtract the counts in the KCl-treated lanes from the KOH-treated lanes. Calculate the sum of counts for all bands. The relative ribonucleotide incorporated at a specific position (Fig. 1c) is calculated as the pixel counts in one band divided by the total number of scintillation counts loaded in the gel lane.
3.2 Strand-Specific Probing for Alkali-Sensitive Sites in Yeast Genomic DNA
3.2.1 Isolation of Genomic DNA
- Isolate yeast genomic DNA using the Epicentre MasterPure Yeast DNA Purification Kit following instructions for harvesting cells from liquid cultures. 50 mL cultures are grown overnight at 30 °C to an OD600 between 0.5 and 1. All steps are performed as described in the kit instructions, with the following modifications:
- RNase A is not included during cell lysis. Following completion of lysis and final resuspension of the DNA in 35 µL TE, 1 µL of 5 µg/µL RNase A is added to the tube and incubated for 30 min at 37 °C. Add 0.1 volumes of 3 M sodium acetate (pH 5.2) and 2.5 volumes of 100 % ethanol, incubate 20 min on ice and microfuge for 20 min at 4 °C at 16,000 × g. Aspirate the supernatant and wash pellet with 500 µL of 70 % ethanol. Dry pellet on bench and resuspend in 35 µL of 1× TE before quantitation.
- Quantitation of DNA is performed using a fluorometric assay (see Note 12).
Following quantitation, precipitate 5 µg of DNA from each sample by incubation with 0.1 volumes of 3 M sodium acetate, pH 5.2 and 2.5 volumes of 100 % ethanol in a total volume of 200 µL. Incubate on ice for 20 min.
Centrifuge for 20 min at 4 °C at 16,000 × g. Dry DNA pellet on bench and resuspend in 20 µL H2O.
Add 6 µL of 1 M KOH (final concentration of 0.3 M) and incubate at 55 °C for 2 h.
Add 4 µL of 6× alkaline DNA loading buffer to each sample.
3.2.2 Alkaline Agarose Gel Electrophoresis
Cast an alkaline agarose gel (see Note 7).
Load 24 µL samples into a 1 % alkaline agarose gel and electrophorese at 30 V for 30 min (until samples have migrated out of the wells). Include a DNA size marker.
Run at 10 V for 18–20 h at room temperature.
Neutralize the gel in Neutralization buffer I for 45 min at room temperature with agitation. Change buffer and repeat for a total of two washes.
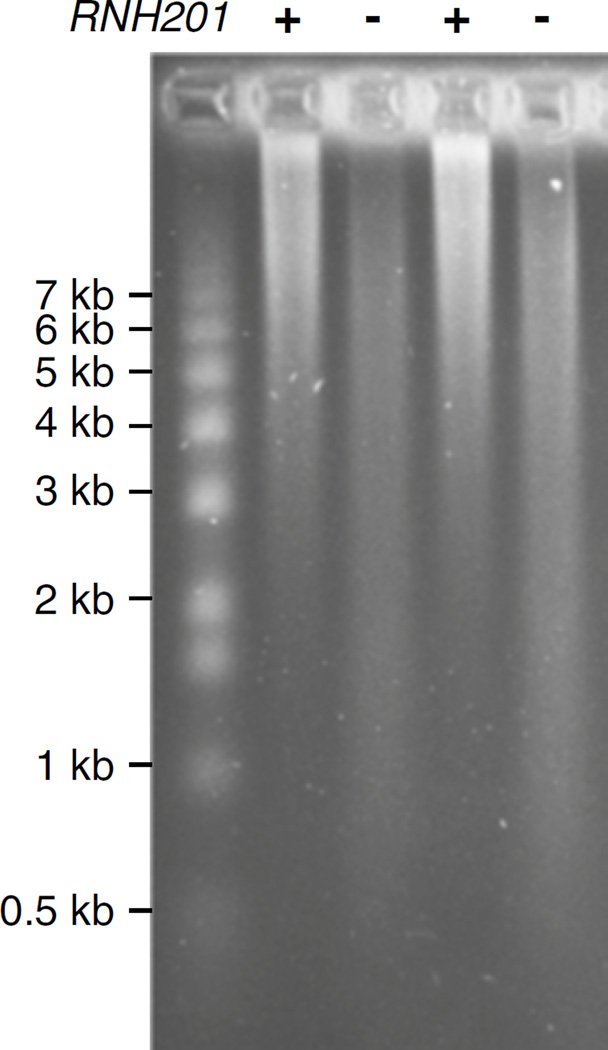
Immerse the gel in H2O and stain using 0.5 µg/mL ethidium bromide for 45 min at room temperature with agitation. Visualize DNA using a UV transilluminator. Destain in H2O for 20 min at room temperature if necessary. An example image of an alkaline agarose gel is displayed in Fig. 2.
Fig. 2.
An example of an alkaline agarose gel stained with ethidium bromide. All strains in this experiment harbor an M644G variant of the leading strand replicase, Pol ε, encoded by the POL2 gene. This pol2-M644G variant has increased capacity to incorporate ribonucleotides in vitro and in vivo [8, 12, 13]. Alkali-sensitive sites in the nuclear genome of rnh201Δ strains (lanes designated (−)) that are deficient in RNase H2 activity indicate the presence of unrepaired ribonucleotides
3.2.3 DNA Transfer to Membrane
DNA is transferred from the alkaline agarose gel to the nylon membrane by capillary transfer, as described [10].
Soak gel for 15 min in alkaline transfer buffer.
Briefly wet nylon membrane with H2O and then soak for 5 min in Alkaline transfer buffer.
Invert the three 100 mL glass beakers in the glass baking dish and set a glass plate on top of them. This is the transfer platform.
Drape the two larger pieces of Whatman paper over the sides and fill glass dish with Alkaline transfer buffer. Smooth out any bubbles by rolling a 5 mL pipette over the surface.
Place agarose gel on top, smooth out bubbles. Surround all edges of the gel with parafilm.
Wet the top of the gel with Alkaline transfer buffer and place the nylon membrane on top, smoothing out bubbles.
Wet 2 pieces of Whatman paper in Alkaline transfer buffer, place on top of membrane, smooth out bubbles.
Place paper towels on top, followed by the glass plate and weight and let transfer overnight at room temperature.
Disassemble transfer stack and soak the membrane in Neutralization buffer I for 15 min at room temperature (see Notes 13 and 14).
3.2.4 Preparation of Strand-Specific Single-Strand Probe
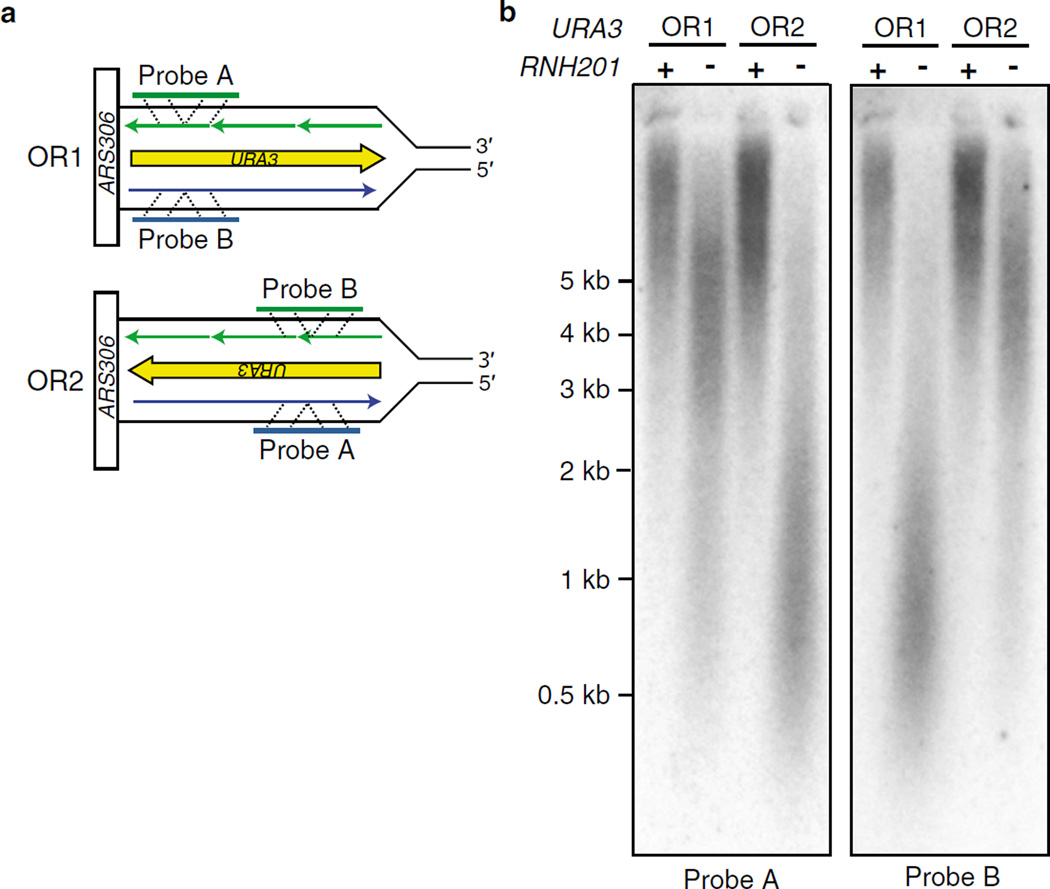
The experimental strategy for strand-specific Southern blotting is based on the approach first described by Carr and colleagues [11]. In our Saccharomyces cerevisiae strains, the URA3 reporter gene is inserted in one of two orientations proximal to a well-characterized, early-firing origin of replication (ARS306) on chromosome III. The origin proximity of URA3 allows the identification of nascent leading and lagging DNA strands using ribonucleotides as a biomarker of DNA polymerase activity (see Note 15) (Fig. 3a).
- The first 520 bp of the URA3 reporter gene is amplified from S. cerevisiae genomic DNA using the following primer pair and a standard PCR reaction (not described here):
- URA3-F1 (5′-GCTACATATAAGGAACGTGCTGC) and
- URA3-R1 (5′-CTTTGTCGCTCTTCGCAATGTC).
Following amplification, purify the PCR product using PCR Purification Kit. DNA is eluted from the column using 50 µL H2O.
-
This product DNA is used as template in the below reaction for radiolabeling of a single strand probe.
For strand-specific labeling, one of the following primers is utilized:- URA3-A (5′-CTCATCCTAGTCCTGTTGCTGCC) for visualizing DNA that anneals to probe A. This corresponds to nascent leading strand DNA when URA3 is in OR2 and nascent lagging strand DNA when URA3 is in OR1.
- URA3-B (5′-CAGTACCCTTAGTATATTCTCCAG) for visualizing nascent DNA that anneals to probe B. This is corresponds to nascent leading strand DNA when URA3 is in OR1 and nascent lagging strand DNA when URA3 is in OR2.
-
Set up the radiolabeling reaction:
Template DNA 10–20 ng PCR-amplified
URA3 fragmentPrimer 2 µL (10 µM stock) 10× buffer 5 µL 2.5 mM dNTPs (minus dCTP) 4 µL α-32P-dCTP 5 µL (50 µCi) TaKaRa Ex Taq DNA Polymerase
(5 U/µL)0.5 µL H2O up to 50 µL. The incubation conditions for the radiolabeling reaction are the following:- Step 1: 94 °C for 5 min
- Step 2: 94 °C for 1 min, 55 °C for 30 s, 72 °C for 1 min for 25 cycles
- Step 3: 72 °C for 5 min
- Step 4: 4 °C forever.
Remove unincorporated nucleotides using a G-25 spin column following manufacturer’s instructions. Spin column 1 min at 720 × g to remove buffer, load radiolabeled reaction product to center of resin, spin for 2 min at 720 × g to elute the labeled probe.
Denature the probe by heating at 95 °C for 5 min before adding to hybridization reaction.
Fig. 3.
(a) Schematic diagram of the orientation of the URA3 reporter gene on chromosome III adjacent to the ARS306 origin of replication. Template strands are in black, the nascent leading strand is in blue and the nascent lagging strand in green. The orientation of the reporter gene with respect to coding sequence is indicated as orientation 1 (OR1) or orientation 2 (OR2). Strand-specific radiolabeled probes that anneal to one of the two nascent strands are designated Probe A and Probe B. Their strand-specificity is dependent on the orientation of URA3. (b) An example of a southern blot probing for alkali-sensitive sites in the nascent leading and lagging strands of yeast genomic DNA. All strains in this experiment harbor the pol2-M644G variant that has increased capacity to incorporate ribonucleotides [8, 12, 13]
3.2.5 Southern Hybridization
Roll the wetted membrane from Subheading 3.2.3, step 9, into a hybridization bottle, add 25 mL hybridization buffer and incubate at 65 °C for 1–2 h with rotation. This is the prehybridization step.
Pour off hybridization buffer. Replace with 25 mL of fresh hybridization buffer and add radioactive probe described under Subheading 3.2.4, step 5.
Hybridize the immobilized DNA to the probe for 16–18 h at 65 °C in a hybridization oven with rotation. Pour off solution into an appropriate radioactive waste container (see Note 3).
Wash 5 times for 5 min each with Phosphate-SDS Washing Solution I at room temperature.
Wash 2 times for 15 min each with Phosphate-SDS Washing Solution II at 65 °C.
Carefully remove the membrane from the hybridization tube using tweezers and lay down on an opened plastic sheet protector sleeve. Cover and expose to an imaging plate. Exposure times will be between 4 h and 96 h. An example of a blot is shown in Fig. 3b.
3.2.6 Stripping the Blot
Incubate the membrane in Blot Stripping Solution for 2 h at 65 °C.
Pour off solution into radioactive waste container, wash 2 times with Phosphate-SDS Washing Solution II for 15 min at 65 °C.
Check the membrane with Geiger counter. If it remains radioactive, repeat all steps of the stripping procedure.
When ready to re-probe, perform prehybridization and hybridization steps as described above (see Note 16).
Acknowledgments
We thank Mercedes Arana and Katarzyna Bebenek for thoughtful comments on the manuscript and Kunkel lab members for technical expertise and discussions. This work was supported by Project Z01 ES065070 to T.A.K. from the Division of Intramural Research of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Prepare fresh APS and formamide loading buffer for each experiment.
0.8 mm spacers and combs are used to purify the unlabeled oligos. Usually, 2–3 wells are needed for each oligonucleotide to obtain reasonable amounts of the purified DNA primer. 0.4 mm spacers and combs are used to isolate full-length radiolabeled products and to visualize products of alkaline hydrolysis.
Dispose of all solid and liquid radioactive waste in appropriate radioactive waste containers.
Metal ion may depend on the polymerase used.
At 1× reactions, these stocks will yield the physiological dNTP and NTP concentrations present in yeast [4].
We prepare this fresh each time.
Heat to dissolve the agarose in water first, cool and then add NaOH and EDTA before pouring into the gel cast. For 100 mL, melt 1 g agarose in 95 mL H2O, cool to 60 °C. Add 5 mL 1 M NaOH and 0.2 mL 0.5 M EDTA, pH 8.0, mix and pour.
Hybridization buffer may need to be warmed prior to use if the SDS precipitates.
Melt the tip of the P200 pipets with a gas flame and flatten the tip before grinding.
Polymerase concentration may depend on the polymerase used.
Add enough radioactive dye on each spot so that counts on the Geiger counter are similar to counts corresponding to the full-length products on the gel. This is necessary to identify both the spots with the hobby dye and the full-length products at the same time.
We use the Qubit 2.0 Fluorometer with the dsDNA BR Assay kit. We find that this is the most accurate quantitation method for DNA prepared using the Epicentre kit.
There is no need to fix the DNA to the membrane for hybridization following alkaline transfer to the positively charged Hybond-N+ nylon membrane.
A membrane that will not be used immediately in a hybridization reaction can be sandwiched between two pieces of Whatman paper and stored at room temperature.
This approach using a target DNA sequence adjacent to an efficient origin of replication should be amenable to other genomic locations as well.
The membrane can be stripped and re-probed approximately three to four times.
References
- 1.Li Y, Breaker RR. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J Am Chem Soc. 1999;121:5326–5372. [Google Scholar]
- 2.Williams JS, Kunkel TA. Ribonucleotides in DNA: origins, repair and consequences. DNA Repair. 2014;19:27–37. doi: 10.1016/j.dnarep.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JA, Suo Z. Unlocking the sugar "steric gate" of DNA polymerases. Biochemistry. 2011;50:1135–1142. doi: 10.1021/bi101915z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhinny SAN, Watts BE, Kumar D, Watt DL, Lundstrom EB, Burgers PMJ, Johansson E, Chabes A, Kunkel TA. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc Natl Acad Sci U S A. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asturias FJ, Cheung IK, Sabouri N, Chilkova O, Wepplo D, Johansson E. Structure of Saccharomyces cerevisiae DNA polymerase epsilon by cryo-electron microscopy. Nat Struct Mol Biol. 2006;13:35–43. doi: 10.1038/nsmb1040. [DOI] [PubMed] [Google Scholar]
- 6.Clausen AR, Zhang S, Burgers PM, Lee MY, Kunkel TA. Ribonucleotide incorporation, proofreading and bypass by human DNA polymerase delta. DNA Repair (Amst) 2013;12:121–127. doi: 10.1016/j.dnarep.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lujan SA, Williams JS, Clausen AR, Clark AB, Kunkel TA. Ribonucleotides are signals for mismatch repair of leading strand replication errors. Mol Cell. 2013;50:437–443. doi: 10.1016/j.molcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElhinny SAN, Kumar D, Clark AB, Watt DL, Watts BE, Lundstrom EB, Johansson E, Chabes A, Kunkel TA. Genome instability due to ribonucleotide incorporation into DNA. Nat Chem Biol. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JS, Clausen AR, Nick McElhinny SA, Watts BE, Johansson E, Kunkel TA. Proofreading of ribonucleotides inserted into DNA by yeast DNA polymerase epsilon. DNA Repair (Amst) 2012;11:649–656. doi: 10.1016/j.dnarep.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook J, Russell DW. Molecular cloning, a laboratory manuel. 3rd. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 11.Miyabe I, Kunkel TA, Carr AM. The major roles of DNA polymerases epsilon and delta at the eukaryotic replication fork are evolutionarily conserved. PLoS Genet. 2011;7:e1002407. doi: 10.1371/journal.pgen.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lujan SA, Williams JS, Pursell ZF, Abdulovic-Cui AA, Clark AB, Nick McElhinny SA, Kunkel TA. Mismatch repair balances leading and lagging strand DNA replication fidelity. PLoS Genet. 2012;8:e1003016. doi: 10.1371/journal.pgen.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JS, Smith DJ, Marjavaara L, Lujan SA, Chabes A, Kunkel TA. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol Cell. 2013;49:1010–1015. doi: 10.1016/j.molcel.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]