Table 7. DFT aided validation for nine randomly selected RP oxides that were predicted to have an NCS ground state structure from ML.
| RP oxides | DFT ground state | NCS ground state (in %) | Predicted space groups from ML [irrep label] |
ΔED | ||||
|---|---|---|---|---|---|---|---|---|
| as predicted from ML | Tree 1 | Tree 2 | Tree 3 | Tree 4 | Tree 5 | |||
| NaLaHfO4 |
P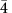 21m (NCS) 21m (NCS) |
100 |
Pca21 [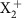 ⊕ ⊕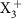 ] ] |
P21212 [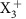 (η1,η2)] (η1,η2)] |
P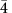 21m [ 21m [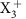 (η1,η1)] (η1,η1)] |
Pca21 [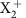 ⊕ ⊕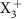 ] ] |
P21212 [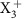 (η1,η2)] (η1,η2)] |
−17.9 |
| NaLaZrO4 |
P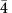 21m (NCS) 21m (NCS) |
80 |
Pca21 [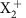 ⊕ ⊕ ] ] |
P21212 [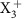 (η1,η2)] (η1,η2)] |
Pbcm [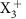 (0,η1)] (0,η1)] |
Pca21 [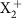 ⊕ ⊕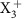 ] ] |
P21212 [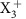 (η1,η2)] (η1,η2)] |
−22.6 |
| NaLaIrO4 (FM) |
P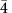 21m (NCS) 21m (NCS) |
100 |
Pca21 [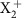 ⊕ ⊕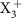 ] ] |
P21212 [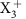 (η1,η2)] (η1,η2)] |
P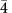 21m [ 21m [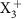 (η1,η1)] (η1,η1)] |
Pca21 [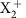 ⊕ ⊕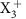 ] ] |
P21212 [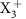 (η1,η2)] (η1,η2)] |
+204.6 |
| KLaIrO4 (FM) | Pbcm (CS) | 80 |
Pca21 [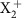 ⊕ ⊕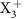 ] ] |
P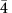 21m [ 21m [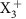 (η1,η1)] (η1,η1)] |
Pca21 [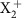 ⊕ ⊕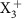 ] ] |
Pca21 [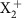 ⊕ ⊕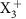 ] ] |
Ibca [P4] | +135.4 |
| KBaNbO4 | P21 (NCS) | 100 |
Pca21 [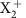 ⊕ ⊕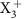 ] ] |
P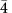 21m [ 21m [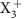 (η1,η1)] (η1,η1)] |
Pca21 [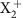 ⊕ ⊕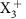 ] ] |
Pca21 [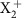 ⊕ ⊕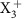 ] ] |
Pca21 [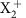 ⊕ ⊕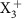 ] ] |
−832 |
| NaCaTaO4 | Pca21 (NCS) | 100 |
Pca21 [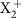 ⊕ ⊕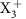 ] ] |
Pca21 [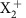 ⊕ ⊕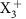 ] ] |
P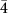 21m [ 21m [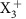 (η1,η1)] (η1,η1)] |
Pca21 [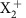 ⊕ ⊕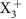 ] ] |
P21212 [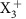 (η1,η2)] (η1,η2)] |
+15.9 |
| SrLaInO4 |
P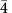 21m (NCS) 21m (NCS) |
100 |
Pca21 [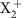 ⊕ ⊕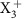 ] ] |
P21212 [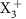 (η1,η2)] (η1,η2)] |
P21212 [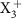 (η1,η2)] (η1,η2)] |
P21212 [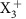 (η1,η2)] (η1,η2)] |
P21212 [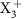 (η1,η2)] (η1,η2)] |
+38.9 |
| SrYGaO4 | P21 (NCS) | 80 |
P21212 [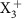 (η1,η2)] (η1,η2)] |
φ |
P21212 [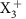 (η1,η2)] (η1,η2)] |
P21212 [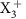 (η1,η2)] (η1,η2)] |
Pca21 [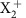 ⊕ ⊕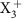 ] ] |
+26.4 |
| BaLaGaO4 | P4/nmm (CS) | 60 |
Pbcm [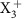 (0,η1)] (0,η1)] |
P21212 [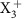 (η1,η2)] (η1,η2)] |
Pbcm [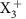 (0,η1)] (0,η1)] |
P21212 [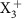 (η1,η2)] (η1,η2)] |
P21212 [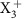 (η1,η2)] (η1,η2)] |
−51.1 |
CS, centrosymmetric; DFT, density-functional theory; FM, ferromagnetic spin order imposed on the Ir-atom; ML, machine learning; NCS, noncentrosymmetric structures.
Note that in a vast majority of compounds the DFT energy difference between space groups P21212 and P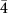 21m is of the order of few tenths of meV per atom. Additional details are given in Supplementary Table 3 and Supplementary Note 4. For KBaNbO4, the structure initialized with Pca21 symmetry converged to P21 in our DFT calculations. ΔED (in meV per atom) is the decomposition energy for a chemical reaction given in Supplementary Note 4. Negative and positive values for ΔED indicate that the compound is thermodynamically stable and unstable, respectively.
21m is of the order of few tenths of meV per atom. Additional details are given in Supplementary Table 3 and Supplementary Note 4. For KBaNbO4, the structure initialized with Pca21 symmetry converged to P21 in our DFT calculations. ΔED (in meV per atom) is the decomposition energy for a chemical reaction given in Supplementary Note 4. Negative and positive values for ΔED indicate that the compound is thermodynamically stable and unstable, respectively.
