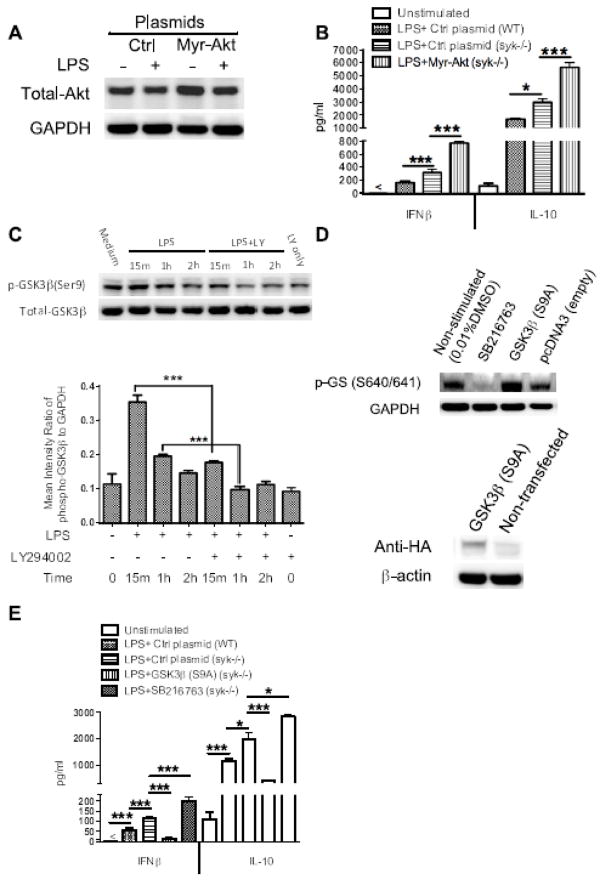
Figure 6. Syk deficiency-mediated elevated IFNβ and IL-10 production is dependent on the activity of Akt-GSK3β signaling.
Syk-deficient cells were transfected with Myr-Akt or pcDNA3-GSK3β (S9A) plasmid which encode constitutively active Akt and GSK3β, respectively. Empty vectors were also transfected into wild type and Syk-deficient cells as a control. After 48 h transfection, cells were stimulated with LPS (1 μg/ml) for 6 h and cells lysates were collected for Western blot analysis. (A) Blot was probed with antibodies to total Akt and GAPDH to ensure equivalent sample loading. (B) After 48 h, transfected cells were stimulated with LPS for 24 h, and then the cell free supernatants were harvested to detect the production of IFNβ and IL-10. (C) Western blot of cell lysates from Syk deficient BMDCs pretreated with PI3K inhibitor, LY294002, for 2 h, then stimulated with LPS to 2 h, the blot was probed with antibodies to phospho-and total GSK3β, densitometric quantification of the ratio of phospho- to total-GSK3β was also calculated as shown. (D) For GSK3β transfection, phosphorylation levels of glycogen synthase (GS), a specific substrate of GSK3β, and the expression level of Hemagglutinin (HA) tag protein, encoded by the HA DNA sequence inserted at the C terminal of GSK3β mutant, were detected by Western blot to assess the transfection efficiency. (E) ELISA of LPS-induced IFNβ and IL-10 in cell-free supernatant in the absence and presence of SB216763 and constitutively active GSK3β. For B and E, data are representative of at least three separate experiments. “*” and “***” indicate p<0.05, and p<0.001 respectively. Data represent the mean ± S.D. of three biological replicates.