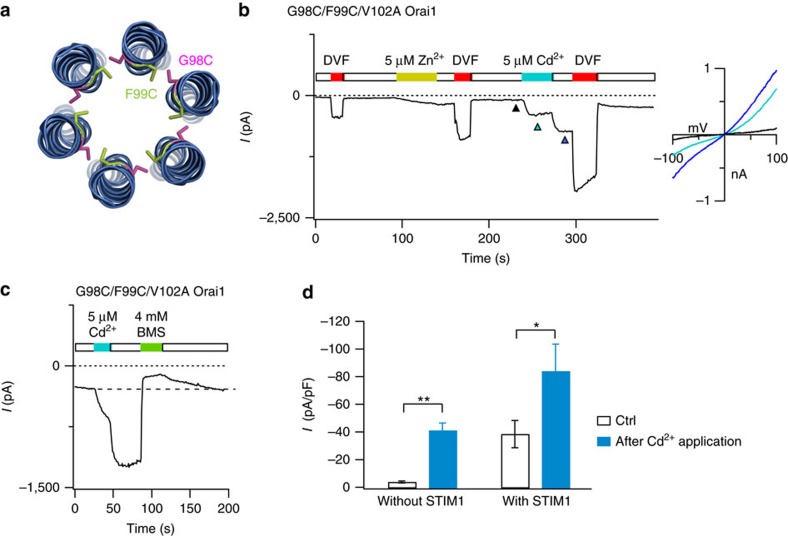
Figure 3. Cd2+ and Zn2+ activate G98C/F99C channels through a lock-open effect.
(a) Cartoon representation of the relative positions of engineered cysteine residues at G98 and F99 in neighbouring subunits based on the crystal structure of Drosophila Orai. The native residues were altered using the mutagenesis tool in PyMOL. (b) The transition metals ions, Zn2+ and Cd2+, strongly enhance currents in G98C/F99C/V102A channels. Potentiation caused by Zn2+ and Cd2+ is partially reversed by washing the cell with DVF solution, indicating that it is caused by a lock-open effect from the trapping of the metal ions in the channel. Note that washout of Cd2+ reveals further current enhancement, indicating that Cd2+ also blocks the potentiated G98C/F99C double cysteine channels probably by binding to a second, low-affinity site. The I–V relationships at the time points indicated by the arrowheads are shown in the right graph. (c) Potentiation of G98C/F99C/V102A current by Cd2+ is rapidly reversed by the reducing agent BMS, likely through destabilization of the thiolate groups trapping Cd2+. (d) Summary of Cd2+ potentiation in G98C/F99C/V102A channels in the absence and presence of STIM1. Cd2+ potentiates G98C/F99C/V102A channels to a greater extent in the absence of STIM1 than its presence, likely because F99C side-chains have already moved away from the pore axis in the presence of STIM1. n=6 cells per condition. **: P<0.001. *: P<0.05. Paired t-test. Values are mean±s.e.m.