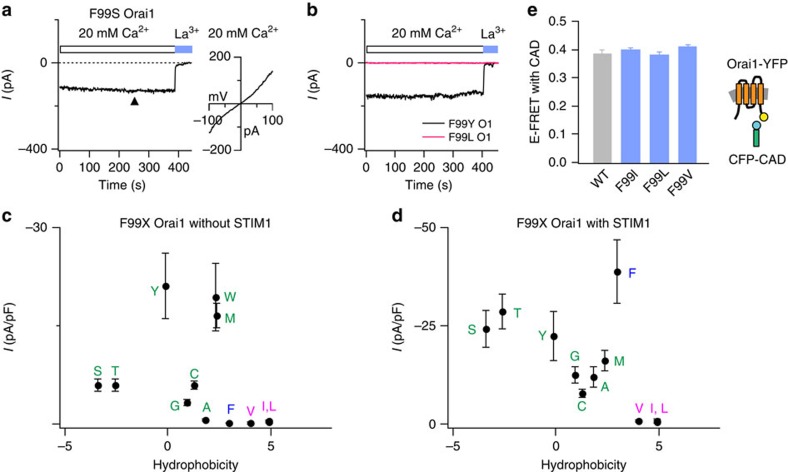
Figure 4. Mutations at F99 produce constitutively permeant Orai1 channels.
(a,b) Time-course of Orai1 current in cells expressing the F99S, F99Y or F99L mutants after whole-cell break-in. Time zero represents the moment of whole-cell break-in. Orai1 mutants were expressed in HEK293 cells without STIM1. The current–voltage relationship of F99S Orai1 at the time point indicated by the arrowhead is shown in the right graph of a. (c,d) Mutational analysis of F99. The current densities measured at steady-state following whole-cell break-in (300–500 s) are plotted against the solvation energies38 (kcal mol−1) of the substituted amino acid as a measure of their hydrophobicity in the absence (c) or presence (d) of STIM1 co-expression. Amino acid hydrophobicity increases from left to right. Values are mean±s.e.m. The native residue (Phe) is shown in blue, the constitutively conducting mutants in green, and the non-conducting mutants in magenta. N=4–6 cells for each mutant. (e) FRET between the indicated Orai1-YFP constructs and CFP–CAD, showing that the mutations do not affect coupling of Orai1 to STIM1. No significant difference was seen in the different mutants compared with WT Orai1. N=32–36 cells for each mutant. Values are mean±s.e.m.