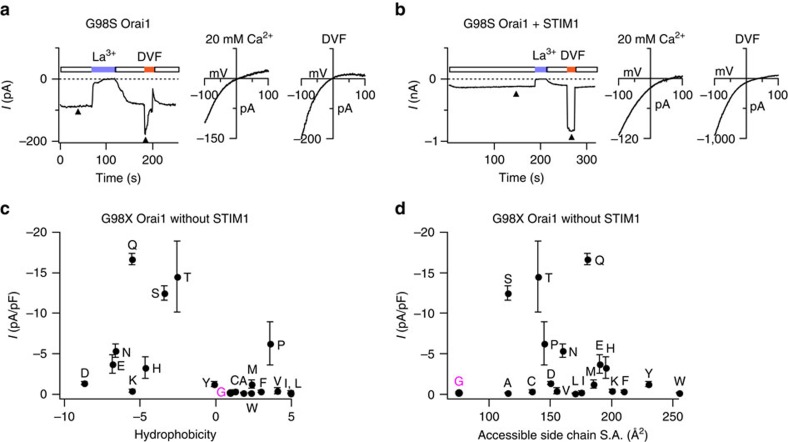
Figure 7. Polar mutations at G98 cause constitutive Orai1 channel activation.
(a) Constitutively active G98S Orai1 current seen following whole-cell break-in. The arrowheads indicate the time points at which the I–V relationships of the current are shown in the right graphs. The I–V plots reveal a non-selective cation current permeable to internal Cs+. G98S Orai1 was expressed in HE293 cells without STIM1. (b) Co-expression of STIM1 alters the ion selectivity of G98S Orai1 channels. The I–V plots in 20 mM [Ca2+]0 and DVF Ringer's solutions (right) show that Vrev is shifted towards positive potentials. (c) Mutations at G98 cause STIM-independent constitutive Orai1 activation. The current densities of the indicated G98X mutant are plotted against the solvation energies38 (kcal mol−1) of the introduced amino acids as a measure of their hydrophobicity in the absence of STIM1. Amino acid hydrophobicity increases from left to right. Values are mean±s.e.m. (d) The constitutive activity of G98X mutants plotted against the accessible side-chain surface area. The native Gly residue is depicted in magenta in c and d. n=3–5 cells for each mutant. Values are mean±s.e.m.