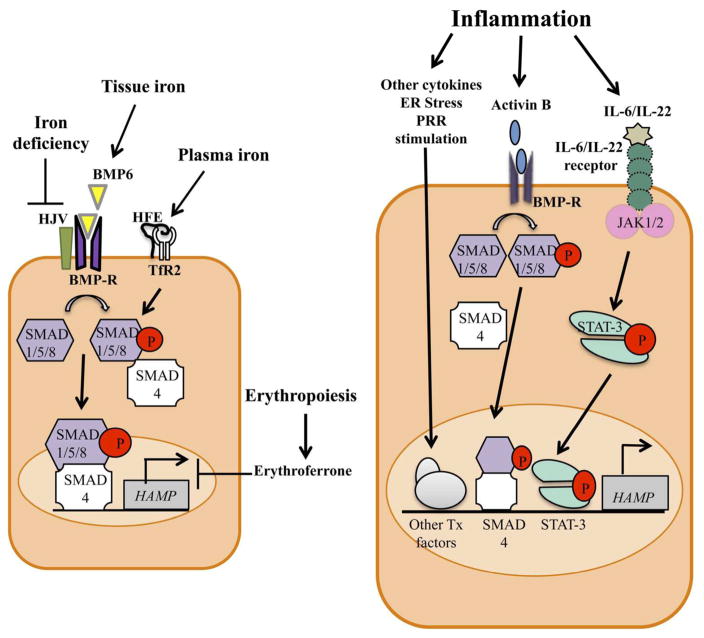
Figure 2.
Regulation of hepcidin expression in hepatocytes in response to iron status and requirements (left) or inflammation (right). Tissue iron increases the expression of bone morphogenetic protein 6 (BMP6), which acts on the BMP receptor (BMP-R) and co-receptor hemojuvelin (HJV). This interaction leads to the phosphorylation of the receptor-associated small-mothers against decapentaplegic (SMAD) 1/5/8 proteins, which then bind to SMAD4, translocate to the nucleus and up-regulate transcription of the HAMP gene to produce hepcidin. Plasma iron is sensed by the hemochromatosis protein HFE and the type 2 transferrin receptor (TfR2), leading to modulation of BMP/SMAD signals. Iron deficiency inhibits BMP/SMAD signals by inducing degradation of HJV, while erythropoiesis inhibits hepcidin expression through the action of erythroferrone. In inflammatory states, cytokines such as IL-6 and IL-22 act on their respective receptors to activate the Janus kinases (JAK)1/2, leading to phosphorylation and dimerization of signal transducer and activator of transcription 3 (STAT3). Dimeric STAT3 translocates to the nucleus to up-regulate HAMP transcription and increase hepcidin production. Activin B produced during inflammation increases HAMP transcription by activating the BMP/SMAD pathway. Additional inflammatory mediators, including other pro-inflammatory cytokines such as IL-1β, endoplasmic reticulum (ER) stress and agonists of innate pattern recognition receptors (PRRs), induce signals that activate other transcription (Tx) factors and up-regulate HAMP transcription.