Figure 1.
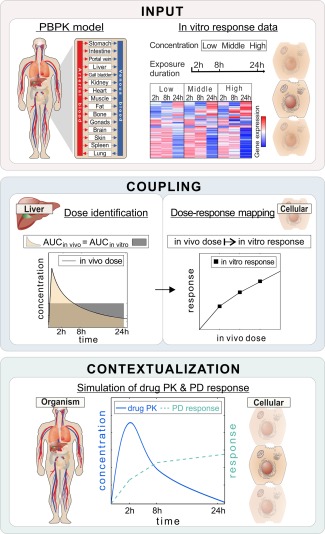
Basic concept of PBPK‐based in vivo contextualization of in vitro toxicity data (PICD). INPUT: Drug‐specific physiologically based pharmacokinetic (PBPK) models are developed at the organism level, whereas in vitro response data of compound‐treated primary hepatocytes are analyzed at the cellular level.28 COUPLING: In vivo doses are identified that are directly related to in vitro drug exposure (area under the curve (AUC)in vivo = AUCin vitro). Time‐dependent dose‐response curves are generated by mapping in vivo doses to in vitro response data. CONTEXTUALIZATION: pharmacodynamic (PD) responses over time are predicted for simulated pharmacokinetic (PK) profiles following drug administration of specific dose levels by use of time‐dependent dose‐response curves.
