Abstract
EEG studies of wakeful rest have shown that there are brief periods in which global electrical brain activity on the scalp remains semi-stable (so-called microstates). Topographical analyses of this activity have revealed that much of the variance is explained by four distinct microstates that occur in a repetitive sequence. A recent fMRI study showed that these four microstates correlated with four known functional systems, each of which is activated by specific cognitive functions and sensory inputs. The present study used high density EEG to examine the degree to which spatial and temporal properties of microstates may be altered by manipulating cognitive task (a serial subtraction task vs. wakeful rest) and the availability of visual information (eyes open vs. eyes closed conditions). The hypothesis was that parameters of microstate D would be altered during the serial subtraction task because it is correlated with regions that are part of the dorsal attention functional system. It was also expected that the sequence of microstates would preferentially transition from all other microstates to microstate D during the task as compared to rest. Finally, it was hypothesized that the eyes open condition would significantly increase one or more microstate parameters associated with microstate B, which is associated with the visual system. Topographical analyses indicated that the duration, coverage, and occurrence of microstate D were significantly higher during the cognitive task compared to wakeful rest; in addition, microstate C, which is associated with regions that are part of the default mode and cognitive control systems, was very sensitive to the task manipulation, showing significantly decreased duration, coverage, and occurrence during the task condition compared to rest. Moreover, microstate B was altered by manipulations of visual input, with increased occurrence and coverage in the eyes open condition. In addition, during the eyes open condition microstates A and D had significantly shorter durations, while C had increased occurrence. Microstate D had decreased coverage in the eyes open condition. Finally, at least 15 microstates (identified via k-means clustering) were required to explain a similar amount of variance of EEG activity as previously published values. These results support important aspects of our hypotheses and demonstrate that cognitive manipulation of microstates is possible, but the relationships between microstates and their corresponding functional systems are complex. Moreover, there may be more than four primary microstates.
Keywords: EEG, microstates, cognition, resting-state, functional systems
1. Introduction
Conceptualizations of the brain as a complex network have initiated innovative investigations of brain organization and function (Bullmore and Sporns, 2009; Sporns, 2011). This paradigm shift towards a network-based understanding of the brain has compelled some investigators to revisit a well-established electroencephalography (EEG) technique developed to characterize the phenomenon of brain electric microstates (Lehmann and Skrandies, 1980). Microstates, observed during the recording of EEG, are defined as brief periods of time during which global electrical brain activity remains semi-stable. These transient periods of stability last between 80 and 120 milliseconds (Lehmann and Skrandies, 1980; Lehmann et al., 1998). Each microstate is classified on the basis of its corresponding EEG scalp potential map (Pascual-Marqui et al., 1995; Wackermann et al., 1993). Previous studies revealed that just four microstates explain nearly 80% of the variance of EEG brain activity during wakeful rest, a state in which subjects are awake and alert, but not engaged in a specific task. These four microstates (labeled A, B, C, and D by Lehmann and colleagues) occur in a repetitive sequence within subjects and there is a typical procession of this sequence across healthy controls, regardless of gender—though there are developmental differences (Koenig et al., 2002; Lehmann and Skrandies, 1980; Lehmann et al., 2005; Van de Ville et al., 2010; Wackermann et al., 1993). Furthermore, the spatial and temporal properties of microstates differ across psychiatric and neurological disorders, including schizophrenia (Andreou et al., 2014; Kindler et al., 2011; Koenig et al., 1999; Lehmann et al., 2005; Strelets et al., 2003), panic disorder (Kikuchi et al., 2011), and Alzheimer’s Disease (Strik et al., 1997). In the case of schizophrenia, several microstate abnormalities have been observed in the prodromal phase (Andreou et al., 2014) as well as in both medication-naïve (Lehmann et al., 2005) and chronic (Strelets et al., 2003) patient populations compared to healthy controls, including irregularities in duration and occurrence (Kindler et al., 2011; Strelets et al., 2003), disturbance of sequence (Lehmann et al., 2005), and abnormal topography (Koenig et al., 1999).
The foregoing findings have generated much excitement about the possibility of using microstates to further our understanding of the neurobiological bases of these various psychiatric diseases. Moreover, these results have led to speculation that microstates are fundamental building blocks of cognition, i.e. the underlying brain activity that subserves human cognitive processes (Khanna et al., 2015; Lehmann et al., 1998). This speculation that microstates are elementary cognitive components is based on two features of microstates: (1) their timescale of occurrence coincides with the sub-second range of synchronous firing of large neural networks (Bressler and Menon, 2010; Logothetis et al., 2001; Whittingstall and Logothetis, 2009); and, (2) the covariance of microstates with diseases that are characterized by profound cognitive deficits, such as schizophrenia (Andreasen et al., 1999, 1996; Schmahmann, 2004). One problem with such an assertion, however, is that EEG microstates contain scant anatomical information due to the inherent limitation in spatial resolution of this methodology—i.e., the EEG inverse problem (Grech et al., 2008). To address this issue, Britz and colleagues simultaneously recorded EEG and functional magnetic resonance imaging (fMRI) to investigate the microstate phenomenon and its relationship with functional systems of the resting human brain. Their investigation showed that the four aforementioned microstates correlated with four well-studied functional systems observed in many resting-state fMRI studies: auditory (microstate A), visual (microstate B), partially cognitive control and partially default mode (microstate C), and dorsal attention (microstate D) (Britz et al., 2010; Power et al., 2011; Yeo et al., 2011).
Despite the evidence implicating each microstate with a specific functional brain system and the association between these systems and specific cognitive functions, to our knowledge only one study to date has attempted to alter microstate features through behavioral manipulation. Recently, Milz and colleagues showed that several microstate parameters are affected by visualization and verbalization tasks compared to both wakeful rest and to each other (Milz et al., 2016). Moreover, there is some evidence that microstates affect the perception of sensory stimuli. A recent study demonstrated that awareness of visual stimuli near the perceptual threshold is influenced by the topography of the microstate that occurs just before stimulus presentation (Britz et al., 2014). However, if microstates are true markers of cognitive and psychological function, then they should be modulated by both task demands and sensory inputs. In this study, the goal is to examine the degree to which specific microstates are influenced by cognitive task, in this case serial sevens subtraction, and by altering sensory input to the visual system (i.e., eyes-open vs. eyes-closed conditions). Another goal of the study is to examine the effect of performing a cognitive task on the sequence of microstate transitions, as alterations in microstate sequence have been observed in patients with schizophrenia (Lehmann et al., 2005).
Serial sevens subtraction was selected for the task condition for several reasons. First, there is evidence demonstrating that serial subtraction activates the dorsal attention system (Kazui et al., 2000). The dorsal attention system is thought to be involved in the voluntary control of attention (Klingberg et al., 1997; Mantini et al., 2007; Ozaki, 2011; Posner and Petersen, 1990; Posner et al., 1988). Moreover, serial sevens is used to measure attention in the Mini Mental State Exam (Moore et al., 1980; Smith, 1967), although some have argued that the task is primarily an index of arithmetic skill and not attention (Karzmark, 2000). Finally, such a task can be performed with both eyes-open and eyes-closed, which allowed for the examination of the effects of alterations in visual input.
It was predicted that (1) a task requiring the voluntary control of attention would significantly increase one or more microstate parameters (duration, occurrence, and coverage) for microstate D, which is associated with the dorsal attention system, as compared to wakeful rest; (2) the sequence of microstates would preferentially transition from all other microstates to microstate D during the task condition as compared to rest; and, (3) the eyes-open condition would significantly increase one or more microstate parameters for microstate B, which is associated with the visual system, as compared to eyes-closed rest.
2. Materials and Method
2.1 Participants
Twenty-four healthy young adults were recruited to participate in the study from fliers posted around the campus of Indiana University and the city of Bloomington for payment, as well as from a subject pool of undergraduate students for course credit. All participants provided written informed consent and the study was approved by the Indiana University Institutional Review Board (protocol #0903000109). Exclusion criteria included a history of neurological or psychiatric disorders, a history of chronic substance use, learning disabilities, and head injuries resulting in a loss of consciousness. More than 24 young adults were recruited, but these excluded participants had an insufficient amount of clean data after application of stringent artifact rejection methods (detailed below). The 24 included participants ranged between the ages of 18–35 (9 male, 15 female; mean age = 21.1; SD = 4.5 years).
2.2 Electroencephalogram
EEG was recorded from 61 cortical Ag-AgCl electrodes (International 10–20 cap system; Falk Minow Services/EasyCap, Munich, Germany) at a sampling rate of 1,000 Hz and gain of 10,000. The specific electrodes sites are Fp1/2, Fpz, AF7/8, AF3/4, AFz, F7/8, F5/6, F3/4, F1/2, Fz, FT7/8, FC5/6, FC3/4, FC1/2, FCz, T7/8, C5/6, C3/4, C1/2, Cz, TP7/8, CP5/6, CP3/4, CP1/2, CPz, P7/8, P5/6, P3/4, P1/2, Pz, PO7/8, PO3/4, POz, O1/2, and Oz. During acquisition EEG data were high pass filtered at 0.02 Hz (12 dB/octave), low pass filtered at 300 Hz (8th order elliptic), and amplified with an EPA Sensorsium (Charlotte, NC) bioamplification system. The horizontal electrooculogram (EOG) data were recorded from electrode sites F9 and F10, and vertical EOG data were recorded from electrodes placed on the left superior and inferior orbits. All EEG electrodes were referenced to a single electrode placed on the tip of the nose. EEG data were recorded continuously using NeuroScan Acquire 4.1 software package and impedances were established below 10 kΩ for all electrode sites. Participants were seated in a comfortable chair in an acoustically attenuated and electrically shielded room.
2.3 Procedures
Participants completed a total of four tasks during the experiment: eyes-open wakeful rest (EOR), eyes-closed wakeful rest (ECR), eyes-open serial subtraction (EOSS), and eyes-closed serial subtraction (ECSS). Each task consisted of three separate two-minute trials with a short break between each trial. During the resting tasks, participants were instructed to remain awake and to allow their minds to wander. During the serial subtraction tasks, participants were instructed to count backwards from a large seed number (639, 691, 732, 783, 816, or 885) by sevens silently and to report the number reached at the end of the trial. Restarting from the seed number was permitted if the participant lost his or her place. In order to minimize eye movements during the eyes-open tasks, participants were instructed to fix their gaze on crosshairs in the center of a computer screen. In order to minimize eye movements during the eyes-closed tasks, participants were instructed to fix their gaze on the crosshairs in the center of a computer screen first and then close their eyes while keeping them positioned as if viewing the crosshairs. The order in which subjects completed the tasks was counterbalanced to allow for all possible permutations (twenty four total).
2.4 Signal Processing
The continuous EEG data were segmented into 5 second epochs and down sampled to 128 Hz. Conservative artifact rejection methods were implemented utilizing algorithms in the MATLAB® (Version 2012b, The Mathworks, Natick, MA) toolbox EEGLab (Version 12.0.1.0b, http://sccn.ucsd.edu/eeglab/). Epochs containing artifacts were rejected by use of the following criteria defined in EEGLab: voltage values extending outside ±150 μV, slopes greater than 50 μV across an epoch, and aberrant distributions of voltage values more than 3 standard deviations away from the mean. Data were then visually inspected, and any epochs containing obvious eye-blink artifacts that the algorithms missed were rejected. Following the methods of Koenig et al., 2002, artifact free epochs were bandpass filtered using the EEGLab basic finite impulse response filter to include broadband frequencies between 2–20 Hz, average referenced, and baseline corrected for further analysis.
2.5 Microstate Analysis
Microstate analysis followed exactly the procedures of Koenig et al. (1999). Briefly, the first five clean, processed epochs of each subjects’ EEG data were included in the microstate analysis. Microstates were assigned to one of k predefined classes via the following method. First, a scalp potential map was assigned to all time points at which there was a Global Field Power peak (instantaneous maximum in the EEG field amplitude). Then, an energy minimization algorithm was used to assign all time points in between Global Field Power peaks to one of the two adjacent peak maps (similar to interpolation). Hence, a sequence of scalp potential maps was generated for each data set. Afterwards, a dissimilarity index implementing modified k-means clustering was used to sort the maps into one of the four predefined (model) microstate classes.
The four model microstate classes were designated as the grand mean microstate maps computed by use of eyes-closed resting (ECR) data from all 24 subjects. Also, the grand mean microstate maps for each other experimental condition were computed in order to compare the topographies of the model microstate classes across conditions. Given the similarity of microstate topographies across conditions (see SI Figure 1), the model maps generated from the ECR data were used as the predefined classes for all conditions. See Section 4.3 for a discussion of the one major topographic difference-microstate B (visual) derived from eyes-open serial subtraction data. Finally, the microstate parameters—duration, occurrence, and coverage—and explained variance were calculated for the sorted microstates (see Koenig et al., 2002, Pascual-Marqui et al., 1995, and Wackermann et al., 1993 for further details). Duration is defined as the total time over which temporally consecutive maps were assigned to the same microstate class. Occurrence is defined as the number of times a microstate occurred during a one second period. Coverage is defined as the total percent of the epoch for which a microstate accounted. Explained variance is defined as the variance of EEG activity explained by all four microstates.
2.6 Statistical Analysis
Separate 2 × 2 × 4 repeated measures analyses of variance (rmANOVAs) were conducted for the three microstate parameters (duration, occurrence, and coverage). Each rmANOVA contained one factor for eye condition (open or closed), one factor for task condition (rest or serial subtraction), and one factor for microstate class (A, B, C, or D). For the explained variance tests both eye and task conditions were examined together in a 2 × 2 rmANOVA (since the factor for microstate classes was not required). Post-hoc paired samples t-tests were used to determine significant differences between eye conditions and task conditions when main effects or interactions in the rmANOVA were significant. To minimize the risk of type I errors, Bonferroni correction was applied separately for each rmANOVA because duration, occurrence, and coverage are not independent measures, which Bonferroni correction assumes. Eight rest versus task comparisons were made per rmANOVA (two for each of the four microstates, one for eyes-closed and one for eyes-open). Thus, the thresholds for significance were p<0.05 for the rmANOVAs and p<0.0063 (p<0.05/8) for the post-hoc t-tests.
2.7 Markov Chain Analysis
A Markov chain is a stochastic model that describes the dynamics of a system with multiple states; that is, if the system is in one state at a certain time point, a Markov chain describes the probability distribution of the system either remaining in that state or transitioning to a different state for the next consecutive time point (Grinstead and Snell, 2010). Separate Markov chains were computed for each of the four experimental conditions in order to avoid a priori assumptions about the underlying probability distribution of state changes for each condition (rest vs. task and eyes open vs. closed). Moreover, one set of chains allowed for microstates to remain in their current state between consecutive time points (self-transitions) and the other did not. The reason for performing the analysis with self-transitions allowed is to test the stability of microstates. The logic for analyzing the data without self-transitions is to characterize the pattern of transitions between microstates when they do occur. Thus, two Markov chains were generated (one allowing self-transitions and the other excluding them) for each of the following conditions: eyes-closed rest, eyes-open rest, eyes-closed serial subtraction, and eyes-open serial subtraction. The null model used for within-condition statistical testing assumed transition probabilities are proportional to the relative occurrence of each microstate, following the precedent set by Lehmann and colleagues (Lehmann et al., 2005).
3. Results
Grand mean model microstate maps are displayed at the bottom of Figures 1 and 2 and in the Figure 3 inset. The results from all of the rmANOVAs and post-hoc analyses are detailed below and organized by each parameter tested. See Figure 1 for duration and occurrence, Figure 2 for coverage, and Figure 3 for explained variance; see Table 1 for summary statistics and results from all post-hoc t-tests.
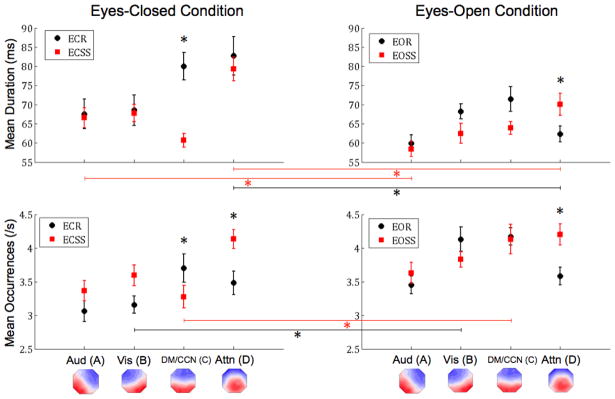
Figure 1. Microstate Duration and Occurrence.
The graphs display the mean duration (top row) and occurrence (bottom row) of each microstate with corresponding standard errors from the mean. The black circles correspond to rest conditions and the red squares correspond to task conditions (serial sevens subtraction). The left column displays results from eyes-closed conditions, while the right column displays results from eyes-open conditions. All significant differences are indicated via asterisks (p<0.0063, Bonferroni Corrected). Lines between columns indicate a comparison between eye conditions. The empirical microstate maps are displayed below and labeled by their associated functional system from Britz et al. (see text for details). EC = eyes-closed, EO = eyes-open.
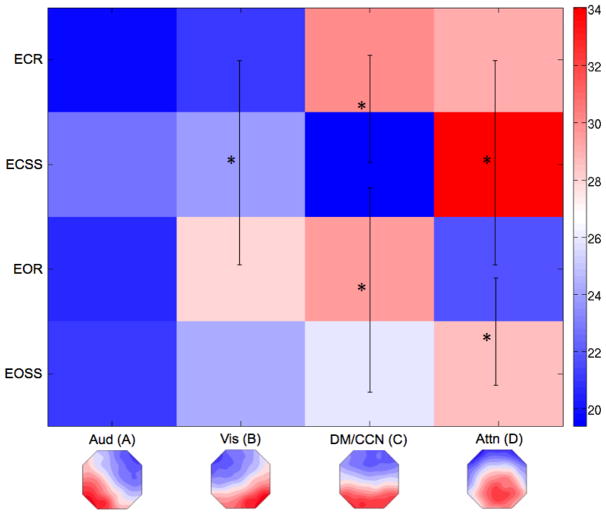
Figure 2. Microstate Coverage (Percent of Total Time).
The matrix displays the total percent of time covered by each microstate for all four experimental conditions. Since the data are percentages, the sum of the values across each row must be 100%. The black lines and asterisks correspond to comparisons that were significantly different (p<0.0063, Bonferroni Corrected). The empirical microstate maps are displayed below and labeled by their associated functional system from Britz et al. (see text for details). EC = eyes-closed, EO = eyes-open.
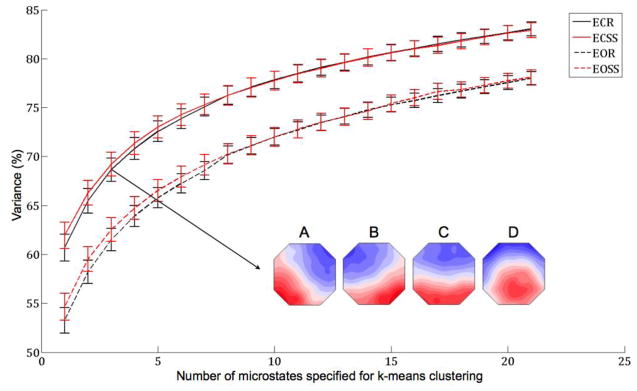
Figure 3. Variance Explained as a Function of Number of Microstates.
The graph displays the percent of variance of EEG activity explained by k number of microstates for k between 2 and 22 microstates, inclusive. The solid lines correspond to eyes-closed conditions and the dashed lines correspond to eyes-open conditions. The black lines correspond to resting conditions and the red lines correspond to task (serial subtraction by sevens) conditions. All comparisons with respect to eye condition were significantly different (closed > open, p<0.0063). The inset displays the empirical grand mean microstate maps derived from all subjects’ eyes-closed resting data with k specified as 4 (methodology of studies by Lehmann, Koenig, and colleagues).
Table 1. Summary statistics and post-hoc tests.
On the left side of the table, means and standard deviations are listed for each microstate by parameter tested (duration, occurrence, coverage, or explained variance) and by experimental condition (eyes-closed (EC) or eyes-open (EO) and rest (R) or task (SS for serial subtraction)). On the right side of the table, p values from the corresponding paired-samples t-tests are reported. Significant values (p<0.0063, Bonferroni Corrected) are displayed in bold.
| ECR | EOR | ECSS | EOSS | Post-hoc comparisons - p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ECR v. ECSS | EOR v. EOSS | ECR v. EOR | ECSS v. EOSS | |
| Duration | ||||||||||||
| A | 64.75 | 15.1 | 60.40 | 10.9 | 67.61 | 13.2 | 58.71 | 9.8 | 0.205 | 0.354 | 0.124 | 0.002 |
| B | 65.29 | 14.0 | 68.63 | 10.1 | 67.71 | 11.3 | 62.47 | 13.0 | 0.396 | 0.036 | 0.226 | 0.085 |
| C | 78.32 | 16.8 | 71.22 | 16.4 | 60.17 | 8.8 | 63.78 | 8.5 | <0.001 | 0.028 | 0.078 | 0.096 |
| D | 81.15 | 18.3 | 62.20 | 10.6 | 81.72 | 15.0 | 69.79 | 14.3 | 0.860 | 0.005 | <0.001 | 0.001 |
| Occurrence | ||||||||||||
| A | 3.09 | 0.80 | 3.47 | 0.65 | 3.34 | 0.78 | 3.62 | 0.80 | 0.065 | 0.265 | 0.016 | 0.015 |
| B | 3.23 | 0.55 | 4.12 | 0.87 | 3.49 | 0.70 | 3.86 | 0.56 | 0.036 | 0.132 | <0.001 | 0.016 |
| C | 3.78 | 0.97 | 4.18 | 0.66 | 3.18 | 0.80 | 4.15 | 1.11 | 0.001 | 0.856 | 0.051 | <0.001 |
| D | 3.64 | 0.78 | 3.57 | 0.69 | 4.16 | 0.73 | 4.19 | 0.78 | 0.004 | <0.001 | 0.758 | 0.917 |
| Coverage | ||||||||||||
| A | 19.79 | 6.1 | 20.71 | 4.4 | 22.67 | 7.1 | 21.02 | 4.8 | 0.048 | 0.722 | 0.505 | 0.228 |
| B | 21.11 | 5.6 | 27.94 | 5.5 | 23.93 | 7.1 | 24.31 | 7.2 | 0.019 | 0.057 | <0.001 | 0.828 |
| C | 29.97 | 10.5 | 29.47 | 6.9 | 19.38 | 6.4 | 25.98 | 5.4 | <0.001 | 0.046 | 0.804 | <0.001 |
| D | 29.15 | 7.3 | 21.89 | 3.7 | 34.03 | 8.4 | 28.68 | 5.2 | 0.015 | <0.001 | <0.001 | 0.016 |
| Variance | 68.65 | 4.8 | 61.50 | 6.3 | 69.20 | 5.3 | 62.53 | 7.2 | 0.441 | 0.639 | <0.001 | 0.002 |
3.1 DURATION
There was a significant interaction effect for eyes x microstate class, F(3,21)=7.686, p=0.001. Post-hoc tests showed that both microstate A (auditory, p=0.002) and microstate D (dorsal attention, p<0.001) had significantly shorter durations during the eyes open condition compared to eyes closed. Additionally, there was a significant interaction effect for task x microstate class, F(3,21)=8.935, p=0.001. Post-hoc tests showed that the duration of microstate C (cognitive control/default, p<0.001) was significantly shorter during the serial sevens task compared to the rest condition. Finally, there was a significant interaction effect between eyes x task x microstate class, F(3,21)=4.591, p=0.013. Follow-up t-tests indicated a significant decrease in the duration of microstate C (p≤0.001) during ECSS compared to ECR. There was also a significant decrease in the duration of microstate D (p 0.001) during all eyes-open conditions compared to the corresponding eyes-closed condition (i.e., EOR < ECR, EOSS < ECSS), as well as a decrease in the duration of microstate A, which was significantly lower (p=0.002) during EOSS compared to ECSS. Overall, microstate duration decreased during the eyes open conditions, resulting in a main effect of eyes, F(1,23)=18.727, p<0.001. Performance of the serial sevens task also decreased microstate duration compared to rest, as demonstrated by main effect of task, F(1,23)=8.722, p=0.007. Lastly, there was a main effect of microstate class, F(3,21)=13.879, p<0.001, for microstate duration, driven largely by microstate D, which follow-up t-tests determined was significantly longer than microstates A and B (p<0.001).
3.2 OCCURRENCE
There was a significant interaction effect for eyes x microstate class, F(3,21)=4.621, p=0.012, which follow up t-tests determined was driven by increased occurrence of microstate B (visual, p<0.001) and microstate C (cognitive control/default, p=0.001) during the eyes open condition compared to when eyes were closed. Also, there was a significant interaction effect for task x microstate class, F(3,21)=12.355, p<0.001, which follow up t-tests showed was primarily due to significantly increased occurrence of microstate D (dorsal attention, p<0.001) during the serial sevens task compared to rest. Finally, there was a significant interaction effect for eyes x task x microstate class, F(3,21)=3.883, p=0.024. Post-hoc analyses indicated that there was a significant increase in the occurrence of microstate B (visual, p<0.001) during EOR compared to ECR and a similar increase for microstate C (p<0.001) during EOSS compared to ECSS. There was also a significant decrease in the occurrence of microstate C (p=0.001) during ECSS compared to ECR and, conversely, a significant increase in the occurrence of microstate D (p<0.001) during EOSS compared to EOR. Overall, microstate occurrence increased during eyes open conditions, F(1,23)=25.303, p<0.001, as well as during the serial sevens task compared to rest, F(1,23)=5.909, p=0.023. Finally, there was a main effect of microstate class, F(3,21)=6.534, p=0.003, for microstate occurrence, in which microstate D occurred significantly more frequently compared to microstate A (auditory, p<0.001).
3.3 COVERAGE
There was a significant interaction effect for eyes x microstate class, F(3,21)=7.219, p=0.002. Follow-up pairwise comparisons showed a significant decrease in coverage of microstate D during the eyes open condition (dorsal attention, p<0.001). There was also a significant interaction effect for task x microstate class, F(3,21)=13.457, p<0.001. Post-hoc tests showed that coverage of microstate C decreased significantly (cognitive control/default, p<0.001) during the serial sevens condition, while coverage of microstate D significantly increased during the serial sevens task (p<0.001). Finally, there was a significant interaction effect for eyes x task x microstate class, F(3,21)=6.384, p=0.003. Post-hoc analyses revealed a significant increase in the coverage of microstate B (visual, p<0.001) with a contrasting significant decrease in the coverage of microstate D (p<0.001) during EOR compared to ECR. Moreover, there was a significant increase in the coverage of microstate C (p<0.001) during EOSS compared to ECSS. There was also a significant decrease in the coverage of microstate C (p<0.001) during ECSS compared to ECR, and a significant increase in the coverage of microstate D (p<0.001) during EOSS compared to EOR. Overall, there was a main effect of microstate class, F(1,23)=11.109, p<0.001, driven by an increased coverage for microstate D compared to microstate A (auditory, p<0.001).
3.4 EXPLAINED VARIANCE
Only a main effect of eyes was observed for EEG variance explained by microstate, F(1,23)=34.126, p<0.001; no significant interaction effects were observed. Post-hoc analyses revealed significantly lower explained variance during eyes-open conditions compared to eyes-closed conditions, regardless of task (rest p<0.001; serial subtraction p=0.002). However, the variance of EEG activity explained by the four microstates during ECR (the canonical methodology for the study of microstates) was lower than values previously reported (μ ± σ = 68.65 ± 4.8% compared to ~80%). Thus, further analyses were performed in order to investigate the amount of variance that is explained by different numbers of microstates (i.e. specifying different values of k during the modified k-means clustering). These analyses were inspired by analyses originally performed by Pascual-Marqui on both modeled EEG data and event-related potential data obtained from an auditory oddball task (Pascual-Marqui et al., 1995). There was a clear increase in explained variance as the number of microstates increased for all experimental conditions. During ECR, the mean explained variance reached a value of 80% or more only after 15 or more microstates were specified for the clustering algorithm (k≥15). In other words, 15 or more microstates were required to explain at least 80% of the variance of EEG activity. The pattern of lower explained variance during eyes-open conditions, regardless of task, remained statistically significant for all values of k tested (2–22).
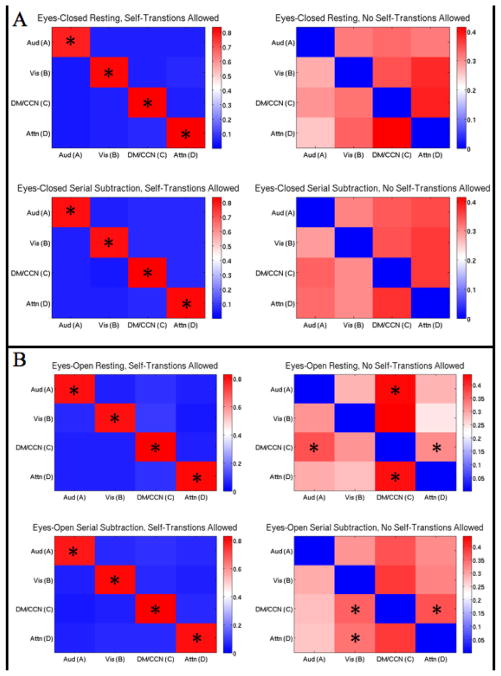
3.5 SEQUENCE OF MICROSTATE TRANSITIONS
The results of the Markov chain analyses are displayed in Figure 4. There was an extremely high probability (~0.8) of self-transitions when allowed, regardless of experimental condition. When self-transitions were not allowed (in order to examine the pattern of between microstate transitions), there were neither discernable nor significant differences in transition probabilities within or between eyes-closed rest and eyes-closed serial subtraction. However, there was a clear preference for transitions to microstate C (from A and D) during eyes-open rest compared to a null model where transition probabilities are proportional to relative microstate occurrence (p<0.0063). This pattern (preferential transitions to C) disappeared during eyes-open serial subtraction and, for microstate D, was replaced with preferential transitions to microstate B. Moreover, microstate C preferentially transitioned to A and D during eyes-open rest and to B and D during eyes-open serial subtraction. Despite the observed significant differences within eyes-open rest and within eyes-open serial subtraction, direct comparisons between rest and task revealed no significant differences for either eye condition.
Figure 4. Sequence of Microstate Transitions.
Each matrix displays the Markov chain for one of the four experimental conditions for all four primary microstates. If the system is in a state at one time point (e.g., microstate A), the matrix displays the probability distribution that the system transitions to another state (e.g, A to B) or remains in that state (e.g., A to A) for the consecutive time point. Self-transitions (A to A, etc.) were allowed for chains in the left column and were not allowed for chains in the right column. Compared to a null model where transition probabilities are proportional to relative occurrence (Lehmann et al., 2005), all microstates were significantly more likely to self-transition across all experimental conditions. A Right Column: Eyes-Closed Conditions, No Self-Transitions Allowed. There were no significant differences within eyes-closed resting, within eyes-closed serial subtraction, or between rest and task that survived multiple comparison correction (each compared to the aforementioned null model). B Right Column: Eyes-Open Conditions, No Self-Transitions Allowed. Under the same aforementioned null model, microstates A and D were significantly more likely to transition to C, while C was more likely to transition to A and D within eyes-open rest. Within eyes-open serial subtraction, microstate C was more likely to transition to B and D, and D was also more likely to transition to B. As before, no direct comparisons between rest and task were significant after multiple comparison correction. *p<0.0063, Bonferroni Corrected
4. Discussion
The goal of this study was to examine the effects of task performance and the state of the visual system (eyes open vs. closed) on EEG microstates. We hypothesized that (1) a task requiring the voluntary control of attention would significantly increase one or more parameters (duration, occurrence, and coverage) of microstate D (associated with the dorsal attention system) as compared to wakeful rest, (2) the sequence of microstates would preferentially shift from all other microstates to microstate D during the task as compared to rest, and (3) eyes-open rest would significantly increase one or more parameters of microstate B (associated with the visual system) as compared to eyes-closed rest.
4.1 ATTENTION TASK AFFECTS MICROSTATE D AS PREDICTED
The data mostly support the first hypothesis; that is, one or more parameters associated with microstate D (dorsal attention) would increase during the serial sevens subtraction task. All three parameters associated with microstate D (duration, occurrence, and coverage) were significantly higher during the task compared to wakeful rest during eyes-open conditions. Similar changes were observed during eyes-closed conditions (task > rest), but due to multiple comparison correction, only occurrence was significantly higher (coverage was at trend levels). These results suggest that cognitive manipulation of a microstate is possible. However, the success of the manipulation may depend on the state of the visual system, since the largest effects were observed during eyes-open conditions (although, the results observed during eyes-closed conditions were in the hypothesized direction). Furthermore, the abovementioned results partially support the association between microstate D and the dorsal attention system reported by Britz and colleagues. The implemented cognitive task, which is thought to activate the dorsal attention system, affected the parameters of microstate D, which is correlated with the dorsal attention system, a functional system activated by attention tasks.
It is important that our results are discussed in the context of the aforementioned work by Milz and colleagues, who examined microstate parameters under three different eyes-closed task conditions. Where we observed an increase in microstate D occurrence during eyes-closed task compared to rest, they observed a decrease in microstate D occurrence during one of their tasks compared to rest. Similarly, where we observed a trend level increase in microstate D coverage during eyes-closed task compared to rest, they observed a decrease in microstate D coverage during two of their tasks compared to rest. However, note that the tasks employed by Milz and colleagues are quite different from the serial subtraction task used here. All of their tasks start with the subject viewing an image, then the subject closes his or her eyes, and finally the subject is asked to concentrate on the image for 50 seconds. The images were either pictures (object visualization), an array of black dots on a white background (spatial visualization), or the text “Define: ‘Familiarization’” with the word to be defined varying across trials (verbalization). There is a clear difference between these tasks and serial sevens subtraction, yet both paradigms require the voluntary control of attention. Hence, one might expect that both behavioral manipulations would have similar effects on the parameters of microstate D. Thus, the association between microstate D and the dorsal attention system may be tenuous. It is also possible that cognitive manipulation of a target microstate is not straightforward, a possibility discussed below.
4.2 ATTENTION TASK AFFECTS MICROSTATE C UNEXPECTEDLY
Although the task manipulation affected microstate D as predicted, the manipulation was non-specific, affecting other microstates in addition to D. Primarily, all three parameters (duration, occurrence, and coverage) associated with microstate C were significantly lower during the task compared to rest (eyes-closed conditions only; trends for duration and coverage during eyes-open conditions). In this case, our results agree with the findings of Milz et al. as they observed a decrease in microstate C duration for two of their tasks compared to rest. Moreover, there was a significant preference for transitions to microstate C from all other microstates during eyes-open rest (a pattern that disappears during eyes-open serial subtraction). These unexpected findings with respect to microstate C may be explained in the context of the task-positive and task-negative systems observed in the fMRI literature.
Specifically, a large portion of fMRI literature argues that the dorsal attention system is task-positive, meaning Blood Oxygen Level Dependent (BOLD) signals localized to the dorsal attention system increase during task performance compared to rest (Petersen and Posner, 2012; Posner and Petersen, 1990). This argument is in accordance with our finding that microstate D parameters increase during the task compared to rest. Note that the dorsal attention system is one among a number of task-positive systems, e.g. fronto-parietal system, cingulo-opercular system (Dosenbach et al., 2008, 2007; Fair et al., 2007); however, there is only one task-negative system observed in the fMRI literature—the default mode network (DMN). BOLD signals localized to the DMN decrease during task performance compared to rest, and there are anti-correlations between DMN BOLD signals and those from many other functional systems during rest, especially the dorsal attention system (Fox and Raichle, 2007; Raichle, 2015; Raichle et al., 2001; Vincent et al., 2007). Taken together, these data suggest that microstate D is task-positive and microstate C is task-negative.
Such an interpretation of microstate C may seem straightforward in the isolated context of the present study, but this claim must be reconciled with the findings of Britz and colleagues. The authors show that a portion of the regions correlated with microstate C belong to cognitive control networks, primarily what they call the salience network, in the tradition of Seeley and colleagues (Seeley et al., 2007), but what others would call the cingulo-opercular system (Coste and Kleinschmidt, 2016; Dosenbach et al., 2008, 2006; Nelson et al., 2010; Neta et al., 2014; Sadaghiani and D’Esposito, 2015; Sadaghiani et al., 2010). Moreover, they make the specific point that in their study none of the four microstates correlated with the DMN. It is worth noting that Britz and colleagues used independent component analysis to confirm the identities of the resting-state functional systems (which were identified by a convolution of EEG and fMRI time courses using a general linear model-see Britz et al., 2010 for details). Therefore, the individual portions of each independent component assumed to form a resting-state functional system are merely spatially independent regions of the brain that share BOLD signal covariance (i.e., the pairwise relationships between the regions are ambiguous). Even so, several previous studies have identified similar components to those Britz and colleagues correlated with microstates (Beckmann et al., 2009, 2005; Smith et al., 2009) and these components are quite similar to functional systems identified via techniques that do provide information about pairwise relationships between brain regions (Dosenbach et al., 2007; Power et al., 2011; Yeo et al., 2011). Thus, system definition is likely not the source of the aforementioned discrepancy.
The cingulo-opercular system is a well-studied functional system that is thought to be crucial for task performance (Coste and Kleinschmidt, 2016; Dosenbach et al., 2008, 2006; Nelson et al., 2010; Neta et al., 2014; Sadaghiani and D’Esposito, 2015; Sadaghiani et al., 2010; Seeley et al., 2007). Several fMRI studies demonstrate that the cingulo-opercular system is involved in the maintenance of behaviorally-relevant task parameters, suggesting that this system supports task-level control (Dosenbach et al., 2008, 2006; Nelson et al., 2010; Neta et al., 2014); however, others would argue that the system is responsible for the maintenance of alertness during task performance and of cognitive resources available for task performance (Coste and Kleinschmidt, 2016; Sadaghiani and D’Esposito, 2015; Sadaghiani et al., 2010; Seeley et al., 2007). Regardless of its specific functions, if microstate C truly represents this system, then one would expect that performance of the serial sevens subtraction would increase the parameters associated with microstate C, since the task requires sustained task performance; however, our data contradict this expectation, as the parameters of microstate C decrease during the task. On the other hand, if microstate C correlates with the DMN, an association supported by our results, then the abovementioned claim may be valid. Future studies should probe this discrepancy, perhaps by use of a more widely implemented cognitive control task (e.g., the N-back task) or by use of network science techniques to identify functional systems. Furthermore, future work should investigate task effects on microstates C and D in a psychiatric population, as a recent meta-analysis revealed that the resting-state properties of these specific microstates vary in patients with schizophrenia (Rieger et al., 2016).
4.3 VISUAL SENSORY MANIPULATION AFFECTS ALL MICROSTATES AND EXPLAINED VARIANCE
The data partially support the second hypothesis; that is, one or more parameters associated with microstate B (visual) would increase during eyes-open rest compared to eyes-closed rest. Most (2 out of 3) parameters associated with microstate B were significantly higher during eyes-open rest compared to eyes-closed rest (occurrence and coverage). Note that there were no significant differences in microstate B parameters observed during task comparisons, which is congruent with two of the three tasks (both visualizations) from Milz et al. (compared to rest). However, the topography of microstate B changed substantially during eyes-open serial subtraction compared to all other conditions (see SI Figure 1). The topographic difference observed here resembles the topographic difference in microstate B reported by Milz and colleagues in three of their between-condition comparisons (see Figure 6 from Milz et al., 2016): object visualization minus verbalization, object visualization minus spatial visualization, and rest minus verbalization (Milz et al., 2016). This discrepancy may explain why we observed no significant differences in microstate B parameters between task conditions.
Once again, the changes in parameters were non-specific; all other microstates were also affected by eye conditions (during eyes-open conditions: decreased duration for A, decreased duration and coverage for D, and increased occurrence and coverage for C). Interestingly, the changes to microstates C and D were in the opposite direction from one another, providing further support for their potential task-positive and task-negative aspects. Perhaps the most striking result is the substantial decrease in explained variance during eyes-open conditions compared to eyes-closed conditions, regardless of task. Taken together, these results suggest that microstate analyses will likely differ depending upon the state of the visual system. Hence, it may be the case that microstate studies implementing different methodologies with respect to eye conditions cannot be compared.
A potential explanation for the decrease in explained variance is that the perception of visual stimuli that are not present during eyes-closed wakeful rest are sufficiently disruptive to increase the variability of electrophysiological signatures of brain dynamics. However, this is speculative and requires the attention of future studies. An alternative explanation, put forth originally by Kondakor and colleagues, is that visual input increases the number of distributed processes in the brain relative to eyes-closed (awake) conditions (Kondakor et al., 1997), which is consistent with the observed decrease in explained variance during eyes-open conditions. Regardless, it is important to note that the explained variance obtained from the experimental data differs substantially from the values reported by Lehmann and colleagues in their previous works (~69% compared to ~80%).
The abovementioned result suggests that there may be more than four primary microstates, a possibility that was investigated further. Our data show clearly that as the number of microstates specified for the clustering algorithm (k) increased, explained variance increased proportionally, regardless of experimental condition. Moreover, upwards of 15 microstates were required to explain a similar amount of variance to the values published by Lehmann and colleagues during eyes-closed rest. Thus, we argue that there are probably more than four primary microstates. However, it appears as though the number is finite, as the curves in Figure 4 seem to approach an asymptote, albeit slowly. Future studies should attempt to determine the optimal number (or range of numbers) of microstates empirically, perhaps by use of similar methods to those employed by Yeo and colleagues to estimate the number of functional systems (Yeo et al., 2011).
4.4 STABLE SEQUENCE OF MICROSTATE TRANSITIONS DURING REST AND TASK
Finally, the data do not support the third hypothesis. None of the Markov chains revealed preferential transitions to microstate D from the other three microstates during task. This result is somewhat surprising at first glance, but it may be explained simply. When self-transitions were considered, there was an extremely high probability that each microstate would remain in its current state between time points (instead of transitioning to a different microstate), regardless of experimental condition. This finding aligns with the widely-observed phenomenon that microstates are semi-stable at rest, and we add to this observation by showing that they remain semi-stable during task. This may explain why we observed only two significant differences in the sequence of microstate transitions when self-transitions were ignored (that were not already present during rest, i.e. microstate C still made preferential transitions to D during eyes-open rest and task).
We observed a preference for transitions to microstate C during eyes-open rest (from A and D). Moreover, this pattern disappeared during eyes-open task, which provides further support for the task-negative nature of microstate C. Such a finding is the exact pattern of activity one would expect to see in the DMN. Also, we observed a change in preferential transitions to microstate B during eyes-open task (from C and D; the rest pattern of transitions was C to A and D to C). This finding may be explained simply: the increased processing demands imposed by performing the difficult serial subtraction task while attempting to stare at the fixation cross may necessitate revisiting the visual system (associated with microstate B) more frequently. It is important to note that the topography of microstate B changed substantially during the eyes-open task (see SI Figure 1), providing further support for our interpretation of increased processing demands during eyes-open serial subtraction. However, direct comparisons between rest and task revealed no significant differences in the sequence of transitions. Nevertheless, closer examination of microstates reveals many significant differences between rest and task and eyes-open and eyes-closed at the level of individual features, as discussed above in detail.
4.5 CONSIDERATIONS FOR FUTURE DIRECTIONS
The above discussion highlights a number of challenges regarding the measurement of EEG microstates. For instance, what is the “correct” number of microstates? What is the best classification method, and what thresholds should be used? These issues are not unique to microstate analysis; any such cluster-based approach will be limited by the use of thresholds that are ultimately somewhat arbitrary. For example, what is the “correct” number of factors or components to retain for a factor analysis or for a principal component analysis? Sometimes data-driven techniques elucidate the answer to this question (e.g., analysis of the distribution of eigenvalues or of comparison data (Ruscio and Roche, 2012)). However, many cases depend on how much explanatory power is gained by including additional numbers of factors or components (or here, microstates). Perhaps a suitable approach is the practical one, as there is an important distinction between trying to find the “correct” number of microstates (if there is such an entity at all) and trying to find the most interpretable number of microstates for understanding EEG data. In the present study, the extraction of four microstates was motivated by the central hypothesis that manipulation of cognitive demands would impact the parameters of microstates described previously in the literature (Koenig et al., 2002; Lehmann and Skrandies, 1980; Lehmann et al., 2005, 1998) and would further test the hypothesized relationship between microstates and resting-state functional systems (Britz et al., 2010). However, the question of how many behaviorally meaningful microstates can be extracted from EEG (as well as their relationship with functional systems) has not yet been addressed, and remains an important area for future research.
4.6 LIMITATIONS
The most confounding result in the present study is that the empirically computed model map of microstate D is notably different from the corresponding map published by Lehmann and colleagues (see SI Figure 2). Note that microstate D as computed in the present study is possibly an inversion or rotation of approximately 180 degrees from the same microstate described in previous publications. However, the topography of microstate D observed here was stable across all four experimental conditions (see SI Figure 1). Conversely, the topography of microstate B computed from eyes-open serial subtraction data was substantially different from the other experimental conditions. These two results may support the abovementioned possibility that there are more than four primary microstates. Further, they reemphasize the aforementioned issues of microstate classification (i.e., clustering method and sorting criteria). Either way, such a discrepancy brings into question the comparability of this study with any of Lehmann and colleagues’ work. This is a confounding factor that must be considered when discussing any results from this experiment.
Another limitation was a problem inherent to the cognitive manipulation; specifically, the inability to ensure that the subjects were counting during the serial subtraction task. Ideally, only subjects who reported possible correct responses on a majority of trials would be included in the final analyses (possible correct response = seed number – 7n, for any natural number n). However, the task proved to be too difficult to implement such a rejection criterion, as only one subject reported all 6 possible correct responses (mean correct responses = 2.2). Instead, subjects who reported impossible values (e.g., higher than the starting seed number, non-integer numbers, etc.) were excluded from the analyses. We are confident that all of the included subjects were performing the task on the basis of observations and notes taken by the experimenters who collected the data.
Finally, in order to maintain context within the broader body of microstate literature, we limited our analyses to 4 model microstate classes for each experimental condition. Moreover, we processed our data in the same way as other microstate studies (e.g., filtered from 2–20 Hz), but with extremely conservative artifact rejection criteria, which limited the amount of data we were able to include in the microstate analysis (25/120 seconds per condition per subject). However, it is possible that our results would vary if more than 4 microstates were specified for the k-means clustering, under different processing regimes (e.g., considering a wider range of frequencies), or if more data were available for microstate analysis. Perhaps the first is not just possible, but likely given the fact that k=4 microstates explained at most 69% of the variance of EEG activity. Furthermore, the observed effects due to the state of the visual system (i.e., eyes-open vs. eyes-closed), may be related to fluctuations in brain activity related to arousal levels (Horovitz et al., 2008; Tagliazucchi and Laufs, 2014). Microstate analyses are particularly susceptible to this issue given that the frequency range usually considered is dominated by alpha band activity (8–13 Hz), which is known to change between eyes-open and eyes-closed conditions (Berger, 1929). Thus, our interpretations are limited by all of these factors, which need to be addressed carefully by future microstate studies.
5. Conclusions
Cognitive manipulation of microstates is possible under certain conditions, i.e. during a serial sevens subtraction task. However, such a manipulation may not be able to target a specific microstate due to the potential task-negative nature of microstate C. Moreover, microstate parameters are substantially different during eyes-open and eyes-closed conditions, indicating that data from both kinds of studies should not be combined and may not be comparable at all. At the very least, it is imperative that microstate studies report eye conditions. Finally, it is possible that there are more than four primary microstates. The use of k-means clustering restricts analyses by sorting microstate maps into specifically (and always) k groups. Previous studies by Lehmann and colleagues assert that k equals four; however, this experiment suggests that may not be the case. Future studies should investigate this matter further without an a priori hypothesis for the number of microstates. A new method of analysis may be beneficial towards this end, such as that developed by Betzel and colleagues (Betzel et al., 2012) or Gartner and colleagues (Gärtner et al., 2015); however, the latter has engendered debate within the field recently (Koenig and Brandeis, 2016). Additional future directions include replicating the experiment in a population with schizophrenia and moving from a cognitive manipulation to a more robust sensory manipulation of microstates (e.g., EEG alpha photic driving).
Supplementary Material
The four model microstate maps from the present study are compared across experimental conditions (eyes-closed (EC) or eyes-open (EO) and rest (R) or task (SS for serial subtraction)). Note the topographic similarities of microstates A, C, and D across all four conditions, whereas the topography of microstate B changes substantially during EOSS. Microstate analyses do not consider differences in polarity (Lehmann and Skrandies, 1980).
The four microstate maps from Koenig et al., 2002 are displayed in the top row and the four microstate maps from the present study are displayed in the bottom row. Note the topographic differences between the two studies’ microstate D. Microstate analyses do not consider differences in polarity (Lehmann and Skrandies, 1980).
Highlights.
We assess the influence of an attention task and visual input on EEG microstates
The manipulations affect some microstate parameters as hypothesized
We observe unexpected task-related decreases for microstate C
Microstate analyses differ substantially when visual input is present versus absent
Targeted cognitive manipulation of microstates is possible; specificity is limited
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (WPH-grant number 2R01MH074983); the Barry M. Goldwater Scholarship and Excellence in Education Program (BAS-2013 Goldwater Scholar); the Indiana University Science Technology and Research Scholars Program (BAS-Summer 2012 Undergraduate Research Grant); and the Indiana University Hutton Honors College (BAS-Summer 2013 Undergraduate Research Grant).
We thank Isaiah Innis and Adam Coey for their help with data collection. We are grateful to Olaf Sporns, Brian O’Donnell, Jeri Kent, and Sarah Forster for many useful discussions.
Footnotes
Conflicts of Interest: None Declared.
Author Contributions
BAS, MA, MAE, and WPH designed the experiment. BAS, MA, and SCB collected the data. BAS, SCB, and ARB analyzed the data. BAS, SCB, MAE, ARB, and WPH wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. doi: 10.1016/S0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Pnas. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C, Faber PL, Leicht G, Schoettle D, Polomac N, Hanganu-Opatz IL, Lehmann D, Mulert C. Resting-state connectivity in the prodromal phase of schizophrenia: Insights from EEG microstates. Schizophr Res. 2014;152:513–520. doi: 10.1016/j.schres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. doi: 10.1073/pnas.0811879106. [DOI] [Google Scholar]
- Berger H. Uber das Elektrenkephalogramm des Menschen. Eur Arch Psychiatry Clin Neurosci. 1929;87:527–570. doi: 10.1007/BF01496966. [DOI] [Google Scholar]
- Betzel RF, Erickson MA, Abell M, O’Donnell BF, Hetrick WP, Sporns O. Synchronization dynamics and evidence for a repertoire of network states in resting EEG. Front Comput Neurosci. 2012;6:1–13. doi: 10.3389/fncom.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Britz J, Díaz Hernàndez L, Ro T, Michel CM. EEG-microstate dependent emergence of perceptual awareness. Front Behav Neurosci. 2014;8:163. doi: 10.3389/fnbeh.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Coste CP, Kleinschmidt A. Cingulo-opercular network activity maintains alertness. Neuroimage. 2016;128:264–272. doi: 10.1016/j.neuroimage.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach Ra T, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A Core System for the Implementation of Task Sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gärtner M, Brodbeck V, Laufs H, Schneider G. A stochastic model for EEG microstate sequence analysis. Neuroimage. 2015;104:199–208. doi: 10.1016/j.neuroimage.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Grech R, Cassar T, Muscat J, Camilleri KP, Fabri SG, Zervakis M, Xanthopoulos P, Sakkalis V, Vanrumste B. Review on solving the inverse problem in EEG source analysis. J Neuroeng Rehabil. 2008;5:25. doi: 10.1186/1743-0003-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstead CM, Snell JL. Markov Chains, Introduction to Probability 2010 [Google Scholar]
- Horovitz SG, Fukunaga M, De Zwart Ja, Van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzmark P. Validity of the serial seven procedure. Int J Geriatr Psychiatry. 2000;15:677–9. doi: 10.1002/1099-1166(200008)15:8<677::AID-GPS177>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kazui H, Kitagaki H, Mori E. Cortical activation during retrieval of arithmetical facts and actual calculation: A functional magnetic resonance imaging study. Psychiatry Clin Neurosci. 2000;54:479–485. doi: 10.1046/j.1440-1819.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- Khanna A, Pascual-Leone A, Michel CM, Farzan F. Microstates in resting-state EEG: current status and future directions. Neurosci Biobehav Rev. 2015;49:105–13. doi: 10.1016/j.neubiorev.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Koenig T, Munesue T, Hanaoka A, Strik W, Dierks T, Koshino Y, Minabe Y. EEG Microstate Analysis in Drug-Naive Patients with Panic Disorder. PLoS One. 2011;6:e22912. doi: 10.1371/journal.pone.0022912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler J, Hubl D, Strik WK, Dierks T, Koenig T. Resting-state EEG in schizophrenia: Auditory verbal hallucinations are related to shortening of specific microstates. Clin Neurophysiol. 2011;122:1179–1182. doi: 10.1016/j.clinph.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Klingberg T, O’Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb Cortex. 1997;7:465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Koenig T, Brandeis D. Inappropriate assumptions about EEG state changes and their impact on the quantification of EEG state dynamics. Neuroimage. 2016;125:4–6. doi: 10.1016/j.neuroimage.2015.06.035. [DOI] [PubMed] [Google Scholar]
- Koenig T, Lehmann D, Merlo MCG, Kochi K, Hell D, Koukkou M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur Arch Psychiatry Clin Neurosci. 1999;249:205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E, Kleinlogel H, Isenhart R, John ER. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Kondakor I, Brandeis D, Wackermann J, Kochi K, Koenig T, Frei E, Pascual-Marqui RD, Yagyu T, Lehmann D. Multichannel EEG fields during and without visual input: Frequency domain model source locations and dimensional complexities. Neurosci Lett. 1997;226:49–52. doi: 10.1016/S0304-3940(97)00224-3. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Galderisi S, Herrmann WM, Kinoshita T, Koukkou M, Mucci A, Pascual-Marqui RD, Saito N, Wackermann J, Winterer G, Koenig T. EEG microstate duration and syntax in acute, medication-naïve, first-episode schizophrenia: A multi-center study. Psychiatry Res - Neuroimaging. 2005;138:141–156. doi: 10.1016/j.pscychresns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Strik WK, Henggeler B, Koenig T, Koukkou M. Brian electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int J Psychophysiol. 1998 doi: 10.1016/s0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann a. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz P, Faber PL, Lehmann D, Koenig T, Kochi K, Pascual-Marqui RD. The functional significance of EEG microstates-Associations with modalities of thinking. Neuroimage. 2016;125:643–656. doi: 10.1016/j.neuroimage.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Moore PN, Pierce D, Graybill D. Digits of equivalent difficulty in the serial subtraction test. Percept Mot Ski. 1980:940–942. [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010:1–12. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Schlaggar BL, Petersen SE. Separable responses to error, ambiguity, and reaction time in cingulo-opercular task control regions. Neuroimage. 2014;99:59–68. doi: 10.1016/j.neuroimage.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki TJ. Frontal-to-Parietal Top-Down Causal Streams along the Dorsal Attention Network Exclusively Mediate Voluntary Orienting of Attention. PLoS One. 2011;6:e20079. doi: 10.1371/journal.pone.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Segmentation of brain electrical activity into microstates: model estimation and validation. Biomed Eng IEEE Trans. 1995;42:658–665. doi: 10.1109/10.391164. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church Ja, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The Brain’s Default Mode Network. Annu Rev Neurosci. 2015:413–427. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod aM, Snyder aZ, Powers WJ, Gusnard Da, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger K, Hernandez LD, Baenninger A, Koenig T. 15 years of microstate research in schizophrenia - Where are we? A meta-analysis. Front Psychiatry. 2016;7 doi: 10.3389/fpsyt.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio J, Roche B. Determining the number of factors to retain in an exploratory factor analysis using comparison data of known factorial structure. Psychol Assess. 2012;24:282–292. doi: 10.1037/a0025697. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M. Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cereb Cortex. 2015;25:2763–2773. doi: 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Lehongre K, Morillon B, Giraud A. Intrinsic Connectivity Networks, Alpha Oscillations, and Tonic Alertness : A Simultaneous Electroencephalography / Functional Magnetic Resonance Imaging Study. J Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/appi.neuropsych.16.3.367. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The Serial Sevens Test. Arch Neurol. 1967;17 doi: 10.1001/archinte.1982.00340190148022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. MIT Press; 2011. [Google Scholar]
- Strelets V, Faber PL, Golikova J, Novototsky-Vlasov V, Koenig T, Gianotti LRR, Gruzelier JH, Lehmann D. Chronic schizophrenics with positive symptomatology have shortened EEG microstate durations. Clin Neurophysiol. 2003;114:2043–2051. doi: 10.1016/S1388-2457(03)00211-6. [DOI] [PubMed] [Google Scholar]
- Strik WK, Chiaramonti R, Muscas GC, Paganini M, Mueller TJ, Fallgatter AJ, Versari A, Zappoli R. Decreased EEG microstate duration and anteriorisation of the brain electrical fields in mild and moderate dementia of the Alzheimer type. Psychiatry Res. 1997;75:183–91. doi: 10.1016/s0925-4927(97)00054-1. doi: http://dx.doi.org/10.1016/S0925-4927(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Laufs H. Decoding Wakefulness Levels from Typical fMRI Resting-State Data Reveals Reliable Drifts between Wakefulness and Sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Van de Ville D, Britz J, Michel CM. EEG microstate sequences in healthy humans at rest reveal scale-free dynamics. Proc Natl Acad Sci U S A. 2010;107:18179–18184. doi: 10.1073/pnas.1007841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder aZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Lehmann D, Michel CM, Strik WK. Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. Int J Psychophysiol. 1993;14:269–283. doi: 10.1016/0167-8760(93)90041-M. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-Band Coupling in Surface EEG Reflects Spiking Activity in Monkey Visual Cortex. Neuron. 2009;64:281–289. doi: 10.1016/j.neuron.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The four model microstate maps from the present study are compared across experimental conditions (eyes-closed (EC) or eyes-open (EO) and rest (R) or task (SS for serial subtraction)). Note the topographic similarities of microstates A, C, and D across all four conditions, whereas the topography of microstate B changes substantially during EOSS. Microstate analyses do not consider differences in polarity (Lehmann and Skrandies, 1980).
The four microstate maps from Koenig et al., 2002 are displayed in the top row and the four microstate maps from the present study are displayed in the bottom row. Note the topographic differences between the two studies’ microstate D. Microstate analyses do not consider differences in polarity (Lehmann and Skrandies, 1980).