Abstract
Visual impairments are common after traumatic brain injury (TBI) and negatively impact quality of life. We describe a 39 year-old woman with a severe TBI who was evaluated by the inpatient optometry and vision rehabilitation service with findings of complete right homonymous hemianopia and right cranial nerve III palsy with 30° right exotropia (eye turn out) and complete right ptosis (eyelid won’t open). The 30° exotropia advantageously generated 30° of right visual field expansion when the right ptosis was treated with a magnetic levator prosthesis (MLP), which restores eyelid opening. Once opened, the patient utilized visual field expansion derived from a right exotropia to overcome functional impairments caused by right hemianopia. Field expansion improved the patient’s wheelchair mobility and reaching tasks during inpatient therapy. This is the first report of visual field expansion by strabismus facilitated by correction of ptosis. Strabismus should be considered for its potential field expansion benefits when homonymous visual deficits are present, prior to considering patching. A multidisciplinary vision rehabilitation team is well suited to make this determination.
Keywords: Brain injury, hemianopia, strabismus, rehabilitation
Introduction
Damage to the posterior visual pathways from neurological disease or trauma results in loss of vision in each eye on the side opposite the brain lesion termed homonymous visual field loss (HVFL or hemianopia). Hemianopia occurs in 30%1 to 50%2 of strokes, 9%3 to 12%4 of traumatic brain injuries (TBIs), affects walking,5–7 driving,8, 9 independence,7 and quality of life,7, 10 and is unlikely to improve if the hemifield loss is complete and time since vision loss is > 1 month.4, 11, 12 The vision loss is fixed to the position of the eye so if the eyes are scanned towards the blind side, objects in that field can be visually detected. The first line of treatment in rehabilitation is to encourage patients to scan frequently and with greater amplitude towards their blind side.13 Scanning training may improve function; however, the extent of the visual field is unchanged.
A well-studied method for expanding the visual field in hemianopia is using prisms to optically induce strabismus (optical exotropia) in peripheral portions of the visual field.14 However, if a patient has strabismus and hemianopia and the deviated eye points into the blind field, field expansion occurs without the need for prisms (Figure 1). This naturally occurring phenomenon which can be referred to as strabismic field expansion has been reported previously15 and may improve detection of obstacles during mobility.16 However, patients with strabismic field expansion may also have double vision on the good side and there is debate whether strabismus should be treated in these cases.15–19 As hemianopia and strabismus are common visual complications from brain injury20 they often occur together and the potential benefits of strabismic field expansion for acute rehabilitation should be considered.
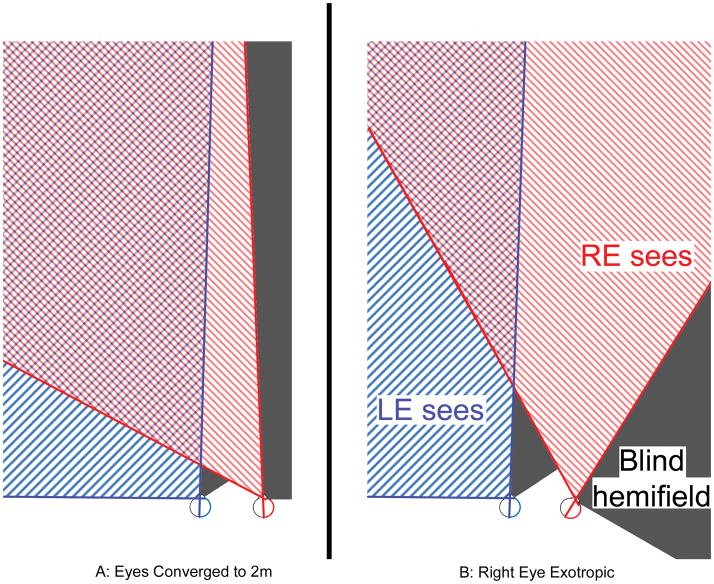
Figure 1.
A: Schematic of right hemianopia when fixating an object 2m distant (cross) and B: field expansion that occurs from right exotropia of 30°. The hashed red and blue lines represent the intact left visual fields. The areas of hemianopic vision loss are shaded gray. Field expansion comes at a cost- overlapping and double images in the portions of the field where red and blue fields overlap. Patients may or may not be bothered by double vision depending on the location of the object of interest, the type of task, and the degree of eye turn. As the magnitude of the eye turn increases, the secondary double image from the deviated eye is shifted further peripherally where it causes less interference. Therefore patients with larger angles of strabismus have more field expansion and are less likely to be bothered by the side effects of the strabismus.
Cranial nerve III palsy is a common cause of strabismus after severe TBI. Patients with traumatic third nerve palsy, which is frequently severe and complete, have a large angle exotropia and hypotropia of the affected eye (eye is down and out), a fixed dilated pupil, and the eyelid is closed (Figure 2a), referred to as paralytic blepharoptosis.
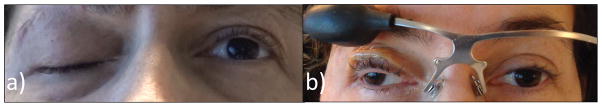
Figure 2.
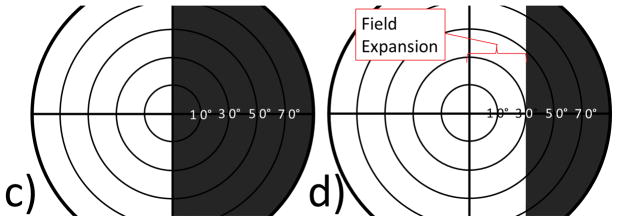
a) Video frame showing complete ptosis of the right eye from CN III palsy. b) When wearing the MLP the right eye can be voluntarily opened and closed. The patient is looking at the camera with the left eye, but direction of gaze of the right eye is approximately 30° rightward due to the CN III palsy/exotropia. c) Illustration of the optometrist drawing from the inpatient record with angular estimates for c) left eye only and d) with the right lid opened with the MLP to reveal the exotropia and field expansion. The dark areas on the plot are unseen, white areas are seen. Confrontations results were identical at the 5-day follow-up.
Ptosis may benefit a patient with CN III palsy, eliminating double vision that would occur were the lid open. However, when hemianopia and CN III palsy occur together, on the same side, the visual field is expanded (Figure 1b). Therefore, if ptosis could be treated, the visual field extent and functional mobility should be immediately improved. For inpatients, a non-surgical treatment is preferable.
A wire on eyeglasses (ptosis crutch) to prop the lid is sometimes used as a non-surgical ptosis treatment, but it does not allow complete eye closure and is inappropriate for patients with motor impairments since it requires continual adjustment.21 A non-surgical prototype device, the magnetic levator prosthesis (MLP), is an improvement over the ptosis crutch.22 It uses externally mounted magnets attached to the eye lid with Tegaderm™ (3M, St. Paul MN) combined with a spectacle magnet system to pull the eye open while still allowing full eye closure, Figure 2b. This system provided safe and comfortable restoration of blinking in 3 patients with severe CN III palsy,22 and 3 patients with severe bilateral paralytic ptosis.23 The MLP is easily disengaged by removing the spectacle frame, yet the lid-magnet may stayed adhered for multiple days without the need for re-application. Because magnetic forces decline rapidly with distance, common metallic objects in the patient’s environment have not caused the spectacles to dislodge.22 Issues of incompatibility with MRI and static magnetic fields can be managed by device removal.
This case report describes a TBI patient with right unilateral CN III palsy and right hemianopia who was treated with the MLP, correcting her ptosis while providing right visual field expansion and improved mobility from the right exotropia. The patient was enrolled in a pilot study for the MLP approved by the institutional review board of Partners Healthcare Human Research Committee, and research adhered to the tenets of the Declaration of Helsinki. Video of each visit was used to record patient responses.
Case Report
The patient was a 39 year-old right-handed Caucasian female with a history of fibromyalgia and asthma who was a motorcycle passenger struck by a car and thrown 40 feet with helmet dislodgement. Her initial Glasgow Coma Scale was 6. She suffered a severe TBI with multifocal intraparenchymal hemorrhage within the bilateral cerebral hemispheres, corpus callosum, bilateral brain stem, and cerebellum consistent with Adams classification Grade III diffuse axonal injury.24 She suffered depressed right orbital floor fracture and comminuted fracture of the right superolateral orbital rim extending to the pterygopalatine fossa without intraorbital or intraconal hematoma or globe injury. She was hospitalized in the neurological intensive care unit for 18 days. Her brain injury was managed with intracranial pressure monitoring and hyperosmolar therapy and her course was complicated by agitation requiring sedation, respiratory failure requiring tracheostomy, and gastrostomy tube for enteral nutrition. She was transitioned to a long-term acute care facility where she progressed from a minimally conscious state to a post-confusional state during her 5-week hospitalization. She was subsequently admitted to an acute rehabilitation hospital.
Initial physiatry evaluation
The patient was awake and alert but restless. She regarded the examiner, followed simple commands but had impaired attention, memory and orientation. She had a dense hemiplegia of the right upper and lower extremity. Signs suggesting right CN III palsy and right hemianopia prompted referral to the inpatient staff optometrist, who specializes in rehabilitation of neurological visual impairments.
Initial optometry evaluation
Eye examination found dense right homonymous hemianopia, total right CN III palsy, and mild-moderate right eye traumatic optic neuropathy. Hemianopia and optic neuropathy were given a poor prognosis for spontaneous improvement. Best-corrected visual acuity in the right eye was reduced to 20/80, the left eye was normal at 20/20. Angle of strabismus was approximately 30° (60 prism diopters) of outward deviation (exotropia) in primary gaze. Confrontations visual fields were performed using a 0.5cm kinetic target and finger-counting method (patient reports number of fingers held in the near periphery). Under monocular conditions the kinetic stimulus was not detected until it crossed into the left hemifield of either eye tested independently, and finger counting ability in the right hemifield of either eye independently was nil. The MLP restored opening and closing of the right eye (Figure 2b), revealing the right exotropia and strabismic field expansion. She immediately reported “being able to see everyone in the room at once,” whereas immediately prior when the right eye was closed she only reported seeing people on her right when directing her gaze to the right. Equivalent to the exotropic angle, a ~30° expansion of the visual field was measurable with the kinetic target (Figure 2c & d), and finger counting in the right field improved to 100% confirming the field expansion was functional. Fixation during confrontations field testing was monitored by the first author confirming the patient was not scanning to her right. When asked if she would like to continue wearing the MLP she responded “I would love to” and reported comfort as 8/10 on a Likert scale, (10 = perfectly comfortable). She did not report double vision during this initial testing.
20-minute optometry follow-up
After a 20 minute trial with the MLP, the patient still rated comfort at 8/10 and denied double vision. She agreed to a 1-week trial, with instructions to wear the device 1–2 hours per day under the direct supervision of her occupational therapist (OT).
5-day optometry follow-up
The OT reported increased independence in activities of daily living (ADL) and mobility with the MLP. Prior to implementation, to facilitate vision, the patient used her left (non-paretic) hand to hold open her right eye, rendering it unavailable for accessing her environment. With the MLP, the OT reported functional improvements in reaching tasks and improved perception of obstacles on her right side during wheelchair propulsion, immediately improving safety and providing independence. Functional independence measures (FIM) confirmed observations. During the initial week of MLP use, FIM scores improved from minimal assistance to supervision with wheelchair mobility, moderate assistance to contact guard for upper body dressing, and moderate to minimal assistance in lower body dressing. Comfort was rated 8/10 commenting that “I feel them [MLP] but they are not bad”, and the patient requested longer wear-times. Eye and skin epithelium showed no ill-effects. She was advised to increase wear time as much as desired. After the 5 day follow-up the patient wore the MLP all day while awake (including therapy and leisure time) without OT supervision.
Rehabilitation Discharge
After 5 weeks, she was discharged home. Her functional mobility improved, ambulating and transferring at a contact guard level with a cane and supervised for dressing. She still had moderate deficits in attention, memory, and problem solving, requiring verbal cues and supervision to maintain safety. Per study protocol, the MLP had to be collected at discharge, thus unfortunately field expansion was lost. A 2-week outpatient optometry re-evaluation was recommended, however the patient was lost to follow-up until 2 months later.
2-month outpatient optometry follow-up
The ptosis had completely recovered and both eyes were open. Exotropia and right hemianopia were still present and essentially unchanged, and field expansion was again evident on confrontations testing. When questioned about double vision she reported it only rarely occurred and was not bothersome. Angular extents of the visual fields were mapped using Goldmann perimetry by an independent technician unaware of the prior results, confirming 30° of field expansion under binocular conditions (Figure 3). The central 20° of the visual field was precisely mapped using an OCT-SLO (Optos, Marlborough, MA) confirming a macular-splitting hemianopia in each eye. Errors in perceived visual direction were observed as rightward misreaching for the examiner’s hand held in the right field expansion area where it was only visible to the exotropic right eye.
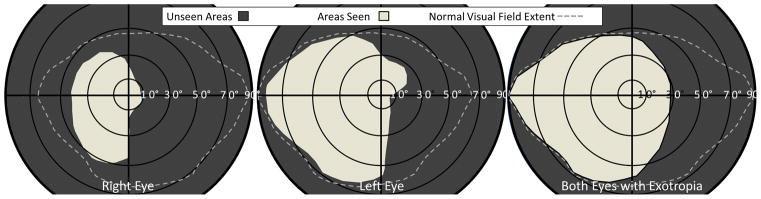
Figure 3.
Goldmann perimetry plots performed 2 months after discharge from the rehab hospital by a technician masked to the expected result. Right visual field expansion out to 30° was measured under binocular conditions (right-most panel), compared to either eye independently (middle and left). Note that the field plots are from the patient’s perspective, whereas the video frames in 2a & 2b are from the clinician’s perspective. Dashed lines represent a normal field extent in people without hemianopia. The right eye had some reduced visual field on the left side as well, related to traumatic optic neuropathy.
Discussion
This is the first report of strabismic visual field expansion in hemianopia facilitated by ptosis correction. Moreover, the inpatient environment allowed documentation of immediate functional improvements. The field expansion was identified during the inpatient stay and carefully measured after discharge by a masked technician. Alternate explanations (residual vision or poor fixation during testing) for the expanded field were unlikely based on the OCT-SLO microperimetry results, which documented good fixation behavior during perimetric testing and complete macular-splitting right hemianopia in each eye. Multiple field measurements over time support the conclusion that field expansion was present, the patient subjectively noted the difference, and the OT observed immediate functional improvements that were lost when the magnetic ptosis glasses (MLP) were removed. Thirty degrees of expansion is only one third of the missing right field, but this central portion of the field is critically important to detect obstacles that pose a collision/fall risk. Strabismus in hemianopia only expands the visual field when the deviated eye points towards the blind hemifield.
Strabismic field expansion might not be tolerable or could negatively impact function because of double vision. For our patient, double vision should have been noticed for objects in the central or left visual field (objects in the blind right field will not be double because they are only seen by the right eye), however this was not the case most of the time. The large magnitude of exotropia and decreased visual acuity in the right eye from the optic neuropathy are likely explanations for minimal double vision symptoms. Had vision between the eyes been equal, or angle of exotropia been smaller, strabismic field expansion may not have been tolerated. In addition to double vision, there is a potential problem from misperception of visual direction related to the strabismus. In exotropia, images seen by the deviated eye are perceived as shifted in the opposite direction of the eye turn. For example, in right exotropia the secondary “double” image appears left of the true image and so obstacles in the right visual field would be perceived as being closer than they actually were. The examiner can observe misperceived visual direction during confrontations testing under binocular conditions by asking the patient to reach for the examiner’s hand held in the expanded hemifield while the patient fixates straight ahead. The patient, if they do not shift their gaze, will misreach an angular extent equivalent to the eye deviation. This behavior confirms field expansion and was observed in our patient. The patient initially required verbal cues from the occupational therapist to shift her gaze to prevent misreaching for objects in the expanded right visual field. This technique helped to facilitate accuracy with reaching tasks. Identical techniques are used when evaluating prismatic field expanding devices for hemianopia, which use optically induced peripheral exotropia (e.g., Peli aka. p-prisms).6, 14
It is also possible that functional improvements reported by the OT were in part related to the beneficial effect of restoring binocular vision on spatial perceptual-attention. Monocular occlusion, whether as a result of ptosis or a patch, can reduce contralateral superior collicular activation, the so-called Sprague effect, inducing spatial bias away from the occluded eye.25–29 Ptosis could therefore have led to reduced rightward orienting and may have improved when use of both eyes was restored, contributing to the observed improvements in function. Analyzing factors that contribute to functional visual impairments allowed the rehabilitation team to administer specific interventions leading to improved function. This patient returned to home and community, which may not have been possible without vision rehabilitation. We caution that utilizing strabismic field expansion could be unsafe if double vision leads to disorientation. In those cases monocular patching, prisms, or strabismus surgery would be considered. Research to identify methods of determining if and when strabismus should be treated in hemianopic patients would be useful to rehabilitation professionals and their patients.
Supplementary Material
Footnotes
Author Disclosures:
A.M. Barrett is employed by the Kessler Foundation, receives honoraria from emedicine, a WebMD company, and receives research funding through the Kessler Foundation from the NIH (R01NS055808, K02 NS047099, K24HD062647), NIDILRR (901F0037) SPR Therapeutics and DART Neuroscience.
Dr. Houston and Paschalis have been involved in the development of the magnetic levator prosthesis but neither they nor their institution (Mass Eye and Ear – Schepens Eye Research Institute) hold financial interest in this device or other technology discussed in this manuscript.
References
- 1.Townend BS, Sturm JW, Petsoglou C, O’Leary B, Whyte S, Crimmins D. Perimetric homonymous visual field loss post-stroke. Journal of Clinical Neuroscience. 2007;14:754–756. doi: 10.1016/j.jocn.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Rowe F, Brand D, Jackson CA, et al. Visual impairment following stroke: do stroke patients require vision assessment ? Age Ageing. 2009;38:188–193. doi: 10.1093/ageing/afn230. [DOI] [PubMed] [Google Scholar]
- 3.Brahm KD, Wilgenburg HM, Kirby J, Ingalla S, Chang C-Y, Goodrich GL. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom Vis Sci. 2009;86:817–825. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- 4.Bruce BB, Zhang X, Kedar S, Newman NJ, Biousse V. Traumatic homonymous hemianopia. Journal of neurology, neurosurgery, and psychiatry. 2006;77:986–988. doi: 10.1136/jnnp.2006.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates JS, Lai SM, Duncan PW, Studenski S. Falls in community-dwelling stroke survivors: an accumulated impairments model. Journal of Rehabilitation Research & Development. 2002;39:385–394. [PubMed] [Google Scholar]
- 6.Bowers AR, Keeney K, Peli E. Randomized Crossover Clinical Trial of Real and Sham Peripheral Prism Glasses for Hemianopia. JAMA Ophthalmology. 2014;132:214–222. doi: 10.1001/jamaophthalmol.2013.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CS, Lee AW, Clarke G, et al. Vision-related quality of life in patients with complete homonymous hemianopia post stroke. Topics in Stroke Rehabilitation. 2009;16:445–453. doi: 10.1310/tsr1606-445. [DOI] [PubMed] [Google Scholar]
- 8.Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia: I. Detection performance in a driving simulator. Invest Ophthalmol Vis Sci. 2009;50:5137–5147. doi: 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers AR, Tant M, Peli E. A pilot evaluation of on-road detection performance by drivers with hemianopia using oblique peripheral prisms. Stroke Res Treat. 2012 doi: 10.1155/2012/176806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorgiou E, Hardiess G, Schaeffel F, et al. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:1749–1758. doi: 10.1007/s00417-007-0644-z. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology. 2006;66:901–905. doi: 10.1212/01.wnl.0000203338.54323.22. [DOI] [PubMed] [Google Scholar]
- 12.Gray C, French J, Bates D, Cartlidge N, Venables G, James O. Recovery of visual fields in acute stroke: Homonymous hemianopia associated with adverse prognosis. Age Ageing. 1989;18:419–421. doi: 10.1093/ageing/18.6.419. [DOI] [PubMed] [Google Scholar]
- 13.Pollock A, Hazelton C, Henderson CA, et al. Cochrane Database Systems Review. John Wiley & Sons, Ltd; 2011. Interventions for visual field defects in patients with stroke (Review) p. CD008388. (008386 pages) [DOI] [PubMed] [Google Scholar]
- 14.Peli E. Field expansion for homonymous hemianopia by optically-induced peripheral exotropia. Optom Vis Sci. 2000;77:453–464. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Herzau V, Bleher I, Joos-Kratsch E. Infantile exotropia with homonymous hemianopia: A rare contraindication for strabismus surgery. Graefe’s Arch Clin Exp Ophthalmol. 1988;226:148–149. doi: 10.1007/BF02173304. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Hoyt CS. To be or not to be: Surgery for exotropia with homonymous hemianopia. Taiwan Journal of Ophthalmology. 2012;2:99–102. [Google Scholar]
- 17.Göte H, Gregersen E, Rindziunski E. Exotropia and panoramic vision compensating for an occult congenital homonymous hemianopia: A case report. Binoc Vis Eye Muscle Surgery Qtrly. 1993;8:129–132. [Google Scholar]
- 18.Hunter DG. Optical factors affecting the outcome of strabismus surgery. Am Orthoptic J. 2003:2–6. [Google Scholar]
- 19.Kerr KE. Anomalous correspondence – the cause of consequence of strabismus? Optom Vis Sci. 1998:17–22. [PubMed] [Google Scholar]
- 20.Rowe F. The profile of strabismus in stroke survivors. Eye (London, England) 2010;24:682–685. doi: 10.1038/eye.2009.138. [DOI] [PubMed] [Google Scholar]
- 21.Lapid O. Eyelid crutches for ptosis: a forgotten solution. Plastic and reconstructive surgery. 2000;106:1213–1214. doi: 10.1097/00006534-200010000-00046. [DOI] [PubMed] [Google Scholar]
- 22.Houston KE, Tomasi M, Yoon M, Paschalis EI. A Prototype External Magnetic Eyelid Device for Blepharoptosis. Transl Vis Sci Technol. 2014;3:9. doi: 10.1167/tvst.3.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houston KE, Tomasi M, Yoon M, Batalha G, Paschalis EI. An External Magnetic Device for Severe Bilateral Blepharoptosis: Proof of Concept (abstract) Optom Vis Sci. 2015:92. [Google Scholar]
- 24.Doyle D, Ford I, Gennarelli T, Graham D, Mclellan D. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 25.Sprague J. interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- 26.Hubel DH, Levay S, Wiesel TN. Mode of termination of retinotectal fibers in macaque monkey-Autoradiographic study. Brain Res. 1975;96:25–40. doi: 10.1016/0006-8993(75)90567-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen P, Erdahl L, Barrett AM. Monocular patching may induce ipsilateral “where” spatial bias. Neuropsychologia. 2009;47:711–716. doi: 10.1016/j.neuropsychologia.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett AM, Crucian GP, Beversdorf DQ, Heilman KM. Monocular patching may worsen sensory-attentional neglect: a case report. Arch Phys Med Rehabil. 2001;82:516–518. doi: 10.1053/apmr.2001.21973. [DOI] [PubMed] [Google Scholar]
- 29.Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. J Rehabil Res Dev. 2006;43:337–346. doi: 10.1682/jrrd.2005.01.0015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.