Figure 3. Mutations at or in proximity to Ser137 can increase or decrease autonomous and Ca2+-CaM stimulated activity.
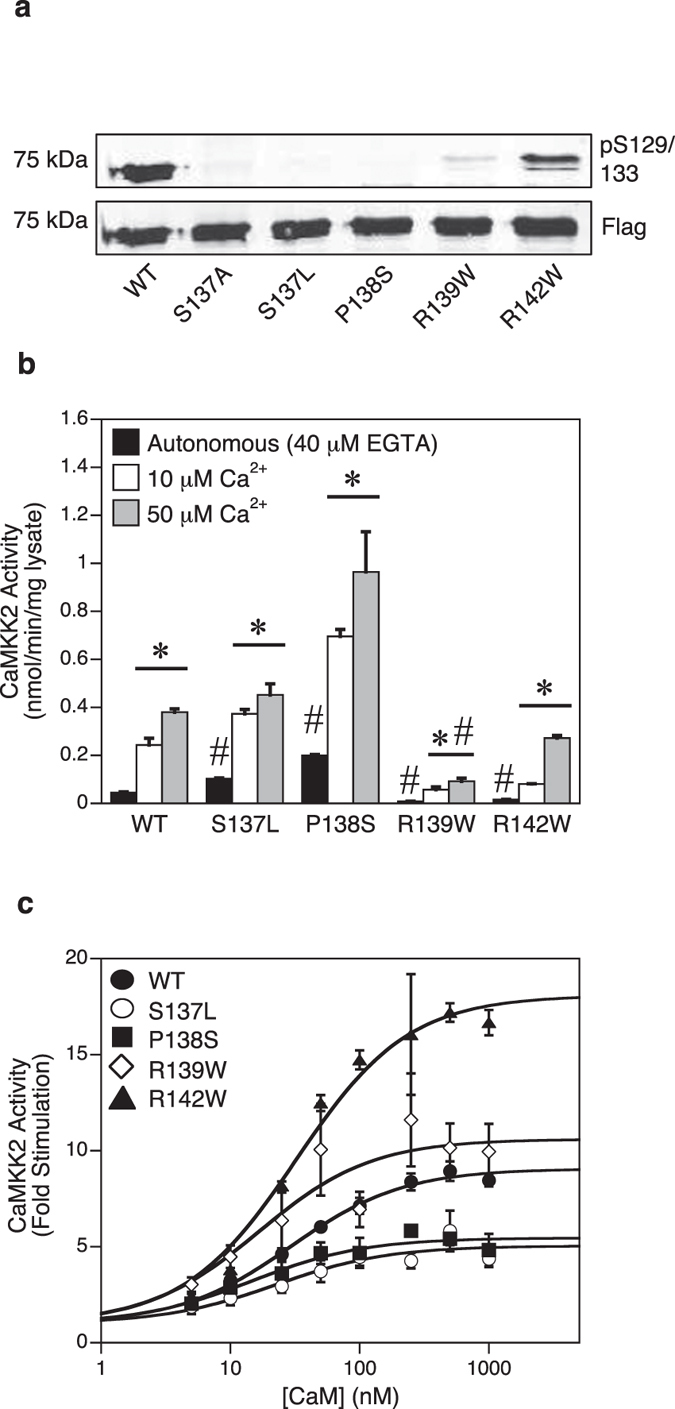
(a) Immunoblot analysis of Ser129 phosphorylation in WT and mutant CaMKK2. Phosphorylation of Ser129 and total CaMKK2 protein were determined (shown on a cropped representative immunoblot) using mouse phosphoserine and rabbit anti-Flag antibodies. The S137A mutant, which we showed previously prevents sequential phosphorylation on the Ser129/Ser133 sites5, was included as a positive control. (b) Autonomous and Ca2+-CaM stimulated activities of WT and mutant CaMKK2 determined in the presence of 40 μM EGTA, 1 μM CaM, 200 μM ATP and either 10 μM or 50 μM Ca2+. CaMKK2 activity was measured using the CaMKKtide peptide substrate assay and the data presented as the mean ± SEM; n = 4–8. (c) Kinase activity of WT and mutant CaMKK2 measured over a range of CaM concentrations (0–1000 nM) in the presence of 50 μM Ca2+. CaMKK2 activity was measured using the CaMKKtide peptide substrate assay and data were fitted to the equation: Activity = Basal + ((((Fold Stimulation × Basal) − Basal) × [CaM])/(A0.5 + [CaM])), where A0.5 is the concentration of CaM giving half-maximal stimulation. Data are presented as mean ± SEM; n = 4–8. Statistical analysis was performed by two-way ANOVA. *p < 0.01, vs the control within the same group; #p < 0.001 vs WT within the same treatment.
