Introduction
The electroretinogram (ERG), a multicomponent electrical signal that can be recorded at the cornea of the vertebrate eye, originates from the responses of retinal neurons to a test flash.1 The first component of the ERG elicited by a brief flash of moderate or high intensity is a cornea-negative response, termed the a-wave, that in mammals reaches its (negative) peak at ~5–20 msec after flash presentation. Abundant evidence indicates that the leading edge (rising phase) of the rod-mediated a-wave closely monitors the massed photocurrent response of the rod photoreceptors, i.e., the flash-induced reduction of rod circulating current.2–11 However, the onset of postreceptor ERG components including the cornea-positive b-wave shapes the a-wave peak and subsequently obscures the rod photoreceptor response (see, e.g., Hood and Birch,12 Robson and Frishman,13 and Wachtmeister14).
The noninvasive nature of ERG recording allows the study of photoreceptor activity in human subjects through analysis of the a-wave response. ERG studies have provided information on the sensitivity (e.g., biochemical amplification) of early activating stages in the rod phototransduction process, and on the maximal excursion of the rod response, under a variety of illumination conditions in both normal subjects and in patients with retinal disease8–10,15–17 (reviewed by Hood and Birch18). However, conventional ERG investigation of the rod photoreceptor response is severely constrained by the shortness of the postflash period that precedes b-wave intrusion. That is, in vitro photocurrent data show that in human rods, as in the rods of other mammalian species, the time scale of the response is several hundred milliseconds with weak flashes and increases with flash strength.19–21 The period of development of the a-wave leading edge, ~20 msec or less, is short in comparison with this overall time scale of the rod response.
The present chapter describes a “paired-flash” ERG method that circumvents the constraint just noted, and in human subjects yields approximate determination of the full time course of the massed rod response to a test flash of arbitrary intensity.22–24 A similar paired-flash method has also been used in studies of in vivo rod responses in experimental animals.25–29
Principle of Paired-Flash Method
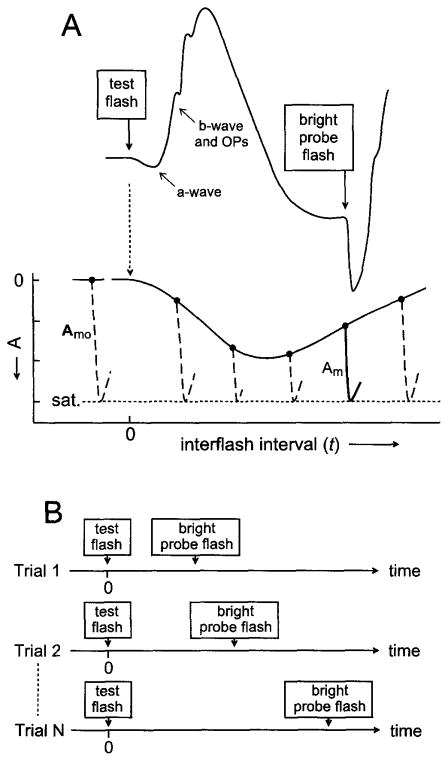
In vitro data from rod photoreceptors show that the burst of cGMP hydrolysis produced by a bright flash leads to a rapid, complete suppression of the circulating current and a resulting maximal, i.e., saturating, photocurrent response (reviewed by Yau30). The paired-flash ERG method described here involves the presentation of a bright probe flash at a defined time after a test flash, and determination of the prevailing response to the test stimulus by analysis of the probe flash response. The central notion underlying the method is that the bright probe flash rapidly drives the rods to saturation and that the amplitude of the probe-generated ERG a-wave titrates the prevailing circulating current. Figure 1A diagrammatically illustrates the concept. For clarity, the cone photoreceptor contribution to the a-wave response is not considered in Fig. 1. The curve in the upper part of Fig. 1A is the hypothetical ERG response to a brief test flash delivered at time zero. Labels identify the a-wave of this response and the subsequent development of the b-wave and oscillatory potentials. Figure 1 further illustrates the later presentation of a brief, high-intensity flash. This second flash, termed the probe, elicits a second ERG response that includes a rapidly developing a-wave. The probe-generated a-wave is presumed to reach its peak within a brief interval that precedes substantial intrusion by the probe-generated ERG b-wave, and the peak of this a-wave response is presumed to correspond with saturation of the rod photocurrent response.
Fig. 1.
Diagrammatic illustration of the paired-flash method. (A) Hypothetical ERG response to a test flash presented at time 0 and to a bright probe flash presented at a later time t. Labels identify the negative-going a-wave and the subsequent b-wave and oscillatory potentials (OPs) of the test-flash-generated response. The secondary ERG produced by the bright probe flash includes a rapidly developing a-wave. The solid hooklike curve in the lower part of the panel reproduces the probe-generated a-wave response of the illustrated ERG. Dashed hooklike curves positioned at t > 0 symbolize probe-generated a-waves obtained by altering the time of probe flash presentation, i.e., by altering the interflash interval t as schematically shown in (B). In (A) the dashed hooklike curve to the left of t = 0 symbolizes the a-wave response to the probe flash presented alone. Peaks of the probe-generated a-waves are aligned at a fixed ordinate value presumed to represent rod photocurrent saturation (sat.). Filled circles represent the baselines from which the probe-generated a-waves depart. The amplitude Am of each probe-generated a-wave is taken as a measure of the rod circulating current at time t; Amo, the maximal amplitude, is determined by the probe-alone response. The measured values of Am(t) and of Amo yield A(t), the derived rod response to the test flash [filled circles and connecting curve; see Eq. (1)]. This conceptual diagram ignores both the contribution of cones to the a-wave responses and the determination of probe-generated a-wave amplitudes at times slightly preceding the response peak. See text for further details.
The lower part of Fig. 1A shows hypothetical probe flash responses (hooklike curves) obtained with variation of t, the time of probe flash presentation, in a series of paired-flash trials. Shown at the left is the hypothetical response to the probe flash delivered in the absence of a recent test flash (“probe-alone” response). These probe responses are positioned with their peaks aligned at the putative constant state representing photocurrent saturation. Filled circles in the diagram represent the baselines from which the probe responses depart. If the prevailing rate of change of the test-flash-induced response at time t is relatively small, little if any change in this test flash response will occur in the brief interval between time t and attainment of the a-wave peak of the probe response. Under these conditions, the peak amplitude of the probe response as referred to the pre-probe baseline will approximate the circulating current at time t. From this determination of the probe response amplitude, and from the maximal amplitude exhibited by the probe-alone response, one obtains by subtraction an amplitude A that represents the rod response to the test flash at time t. That is,
| (1) |
where Am(t) is the probe response amplitude determined in the paired-flash trial, Amo is the amplitude of the probe-alone response, and A(t) is the amplitude at time t of the derived response to the test flash. The family of determinations of A(t) obtained with variation of the interflash interval t (Fig. 1B) yields, in turn, the complete “derived” rod response to the test flash.
The a-wave response to the bright probe flash requires a brief period to reach its peak amplitude. With probe flash strengths of 104 scotopic troland-seconds (sc td-sec) or higher, as in the experiments described below, this period is typically about 8 msec. The procedure just outlined ignores the nonsimultaneity of the probe flash presentation time t and the determination time of the probe a-wave response. That is, determination of the derived response at time t [A(t)] is based on measurement of the probe response amplitude at time (t + ≈8 msec). The error introduced by effectively equating t with (t + ≈8 msec) is negligible when the interflash interval t greatly exceeds 8 msec, i.e., under most of the conditions investigated below. However, with short interflash intervals, consideration of this ≈8-msec period becomes important for accurate representation of the derived response.29 Unless otherwise stated (Fig. 5, below), post-test-flash times quoted for derived response amplitudes presented here are the interflash intervals t.22–24
Fig. 5.
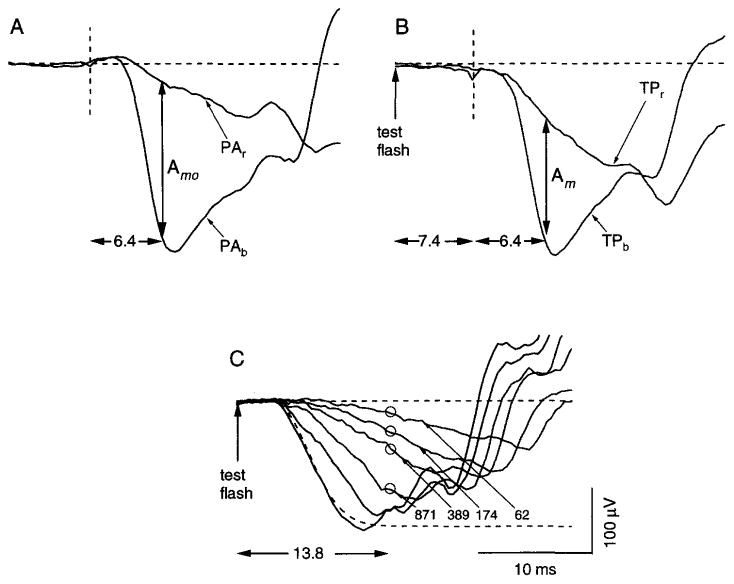
Paired-flash determination of the rod response amplitude with short interflash interval and a relatively strong test flash. Data obtained from a single subject. (A) Responses to the blue probe alone (PAb) and the red probe alone (PAr). Here and in (B), the dashed vertical line is the time of probe flash presentation. The difference between the two responses 6.4 msec after flash presentation (vertical arrow) is taken as Amo, the maximal amplitude of the rod-mediated probe response. (B) Responses obtained in two paired-flash trials, each of which involved presentation of a test flash (174 sc td-sec) and, 7.4 msec later (vertical dashed line), a bright probe flash. The probe used in trial TPb was a bright blue flash; that used in trial TPr was a photopically matched red flash. The difference between the two responses 6.4 msec after probe flash presentation (vertical arrow) was taken as the amplitude Am of the rod-mediated probe response. (C) Rod-mediated a-wave responses to test flashes that ranged from 62 to 3.5 × 103 sc td-sec. The dashed curve represents the fit of a computational a-wave model to the response at 3.5 × 103 sc td-sec. Circles indicate paired-flash determinations of A/Amo at the post-test-flash time of 13.8 msec, obtained for the 174-sc td-sec test flash as described in (B), and similarly obtained for test flashes of 62, 389, and 871 sc td-sec. For illustration with the a-wave data, the value of Amo was equated with the maximal excursion predicted by the a-wave model (asymptote of dashed curve). See text for further details.
Experimental Procedures
General Description
Most of the procedures for obtaining paired-flash ERGs are similar to those for corneal recording of the conventional, i.e., single-flash, ERG. All experiments are conducted in accordance with the principles embodied in the Declaration of Helsinki, and with institutional regulations and guidelines including those for informed consent. Prior to the ERG testing session, the pupil of the eye to be tested is dilated by the application of 1% (w/v) tropicamide and 2.5% (w/v) phenylephrine hydrochloride. An opaque patch is then placed over the eye for 45 min to allow complete dark adaptation. Immediately before the experiment, the patch is removed in the ERG darkroom laboratory, and 0.5% (w/v) proparacaine hydrochloride (topical anesthetic) is applied to the cornea. A bipolar recording electrode (GoldLens; Diagnosys LLC, Littleton, MA), the corneal contact surface of which has been lubricated with 2.5% (w/v) methylcellulose solution, is then placed on the cornea, and a cup-style ground electrode is attached to the forehead.
The subject is seated at a ganzfeld dome that delivers full-field stimuli from two flashguns. Light from one of these two flashguns, representing the test flash, is ordinarily of short wavelength (“blue” flash; Wratten 47B filter; λmax = 449 nm; Eastman Kodak, Rochester, NY) and preferentially stimulates rod photoreceptors. The second flashgun provides the “probe” flash. As needed (see below), the probe stimulus consists of either a short-wavelength flash (“blue” probe; spectrum identical to that of the test flash) or a relatively long-wavelength flash (“red” probe; Wratten 26; λcut-on = 605 nm) that with neutral density attenuation is photopically matched to the blue probe, i.e., is matched to the blue probe with respect to its effectiveness in stimulating cone photoreceptors.9 Light from the probe flashgun also passes through a heat-absorbing filter. Strengths of the flashes associated with a given set of filters are calibrated by the use of an integrating photometer (model 40X; United Detector Technology, Hawthorne, CA). Instrumentation and procedures relevant to recording ERGs from infants are generally similar to those just described. Here the corneal recording electrode is of reduced size (infant GoldLens; Diagnosys LLC), the pupil is dilated with pediatric eye drops, and the subject, in a supine position, views a ganzfeld dome positioned above the head.31
Determination of the time course of the derived response to a fixed test flash, or of the amplitude-intensity relation of the derived response at a fixed interflash interval, requires a series of paired-flash trials. It is important within the series to separate successive trials by a dark-adaptation period to permit the subject to recover completely from the flashed stimuli of the preceding trial. For normal subjects, and for the strengths of the probe flashes used in the experiments described here, we have found a dark-adaptation period of 1 min or longer (depending on the test flash strength) necessary to ensure full recovery. Paired-flash determinations of the incremental rod response to a test flash can also be carried out in the presence of steady background light.24 Background illumination in the ganzfeld dome of our system is provided by two 12-V incandescent bulbs operated at a color temperature of about 2650 K. After the experiment, the rod-mediated component of each probe flash response (see below) is analyzed for amplitude at a fixed time near or somewhat preceding the a-wave peak (typically, 6.4–8.4 msec), and amplitudes of the derived rod response to the test flash are determined from Eq. (1).24 Determinations of stimulus strength in scotopic troland-seconds (flashes) and scotopic trolands (backgrounds) are based on measurement of the diameter of the fully dilated pupil during the experiment.
Additional Technical Considerations
Any of a number of high-intensity photographic units can be used to generate the bright probe flash required in the paired-flash experiments. The probe flash unit of our system consists of a Novatron model 2150 flash head and model 1600 power supply (Novatron, Dallas, TX). The test flash is provided by a second, identical Novatron unit or (for relatively weak test flashes) a Grass photostimulator (model PS-22; Astro-Med/Grass, Quincy, MA). Each of the two flash heads is mounted in a holder that spans a port in the ganzfeld dome. Light from each unit is spectrally shaped (see above) and attenuated (see below) by filters positioned in the holder. The dimensions of the Novatron and Grass flash heads are similar, and the two heads can be readily exchanged. The shot-to-shot variation in flash strength of the Novatron unit is less than 4%. At the maximum power setting, the duration (half-height) of the Novatron flash is about 1.3 msec, i.e., well within the photoreceptor integration time, and the unit is fully recharged within 5 sec. However, the length of this recharge period necessitates the use of two separate flash units, as the interval between the test and probe flashes chosen for a given trial is often far less than 5 sec. The use of separate flash units is made necessary also by the need for independent control of the intensity and wavelength of the two flashes. The neutral density filters used in our early studies were Wratten (gelatin) filters. However, even with the use of an infrared-absorbing filter, gelatin filters are gradually degraded by the heat of the flash unit. Our solution was to prepare a set of “aperture filters” from rectangular sections of Bakelite (thickness, 3 mm), an extremely durable, heat-resistant phenolic. By varying the area of the aperture it was possible to produce a set of filters that, as calibrated by direct measurement of the ganzfeld dome luminance, spanned a convenient range of effective attenuation.
The timing of the flashed stimuli is controlled by a computer equipped with a timing board (PC-TIO-10; National Instruments, Austin, TX). Responses from the ERG electrodes are amplified (model AM-502 differential amplifier; Tektronix, Beaverton, OR) with a bandpass (3 dB down) typically of 2 Hz to 10 kHz. The amplified data are digitized (routinely used sampling rate, 5 kHz) and stored in a computer. The probe flash waveform obtained in a paired-flash trial is acquired over an interval that typically extends to 50 msec after flash presentation. The experimental data are also routinely recorded on tape (bandpass of dc to 9 kHz; model 420 instrumentation recorder; Vetter Instruments, Rebersburg, PA) for later analysis of long-duration signals such as the full ERG response. Near-real-time visualization of the family of probe responses obtained during the experiment is advantageous. For example, variably among subjects and among trials, the test flash elicits a blink reflex at ~70–90 msec after flash presentation that introduces noise into the ERG response. The blink reflex can present a problem for a paired-flash determination when it occurs within the period of recording of the response to the probe flash; visualization of the response immediately after its collection allows evaluation of the need for repetition of the paired-flash trial. With practice, most subjects can learn to minimize this reflex, even to intense flashes. Analysis of the data employs Igor Pro (Wavemetrics, Lake Oswego, OR), a software package compatible with both Windows and Macintosh platforms.
Analysis of Probe Flash Response
Subtraction of Cone Contribution
Paired-flash derivation of the rod response to the test flash depends on accurate determination of the rod-mediated response to the bright probe flash, as the amplitude of this rod-mediated probe response [Am in Eq. (1)] measures the remaining rod circulating current. In the human eye, cone photoreceptors contribute to the a-wave generated by relatively bright flashes; under dark-adapted conditions, this cone contribution represents up to ~20% of the peak amplitude of the overall a-wave response.5,6 Obtaining the derived rod response to the probe flash thus necessitates determining and then subtracting the cone contribution.
A later section (Time Course of Derived Response) describes a “cone subtraction” procedure workable in the case of substantial variation of the cone contribution within a series of paired-flash trials. However, under conditions relevant to two key types of paired-flash experiments, the relatively low photic sensitivity of the cones and their relatively fast recovery kinetics combine to simplify this subtraction procedure. The first of these is determination of the time course of the derived response to a weak test flash, i.e., one for which the peak amplitude of the derived response is well below photocurrent saturation. Here one can expect relatively little cone stimulation by the test flash, and the cone contribution to the bright probe flash of a given paired-flash trial will be essentially constant, i.e., independent of the interflash interval t. The second type of experiment is the determination of recovery kinetics after a strong, i.e., rod-saturating, test flash. In this case the cones are fully recovered by the time of departure of the rods from saturation, and the cone contribution to the probe flash response during the period of rod recovery will also be essentially constant (independent of t). In the two types of experiments just described, each of the series of paired-flash trials routinely employs a short-wavelength (blue) flash of fixed high intensity as the probe stimulus. Also recorded during the experiment is a single waveform obtained in response to a long-wave-length (red) probe flash, the strength of which has been set to achieve a photopic match (equal cone-stimulating activity) with the blue probe flash.5,6,9 The response to the red probe is presumed to represent the (constant) cone contribution to the group of blue probe responses obtained in the paired-flash trials, and is computationally subtracted from each of the blue probe responses to yield the presumed rod-mediated component.
The strength of the blue probe flash used in our experiments is typically set within the range of 104–2.5 × 104 sc td-sec. Figure 2 shows the response to a representative blue probe flash (1.6 × 104 sc td-sec) and illustrates a test of the photopic match being used. Waveform 1 in Fig. 2, which shows the a-wave and subsequent upswing of the response produced by the blue probe flash presented alone (i.e., in the absence of any other recent stimulus), is expected to contain contributions from both the rod and the cone photoreceptors. Waveform 2 is the response produced by a red probe flash of putatively equal photopic strength. These data are compared with waveforms 3 and 4 obtained, respectively, on presentation of the blue or the red probe flash 3 sec after a brilliant conditioning flash that produced a prolonged (about 7-sec) saturation of the rods. The similarity of waveforms 2, 3, and 4 during the ~10-msec period immediately after the probe flash, i.e., throughout the rising phase of waveform 1, indicates that the relatively small responses 2, 3, and 4 are cone-mediated and supports the notion that the red and blue probe flashes are photopically matched. Importantly, the rod-mediated component of the response to the red probe flash is negligible under the present experimental conditions, as the scotopic strength of this red stimulus is only ~1/300 of the scotopic strength of the blue probe flash. That is, for example, the red flash photopically matched to a 1.6 × 104 sc td-sec blue flash has a scotopic strength of ~50 sc td-sec, and, even under dark-adapted conditions, the rod a-wave produced by a flash of this strength is minute during the ~10-msec period relevant to the present analysis of probe flash responses.
Fig. 2.
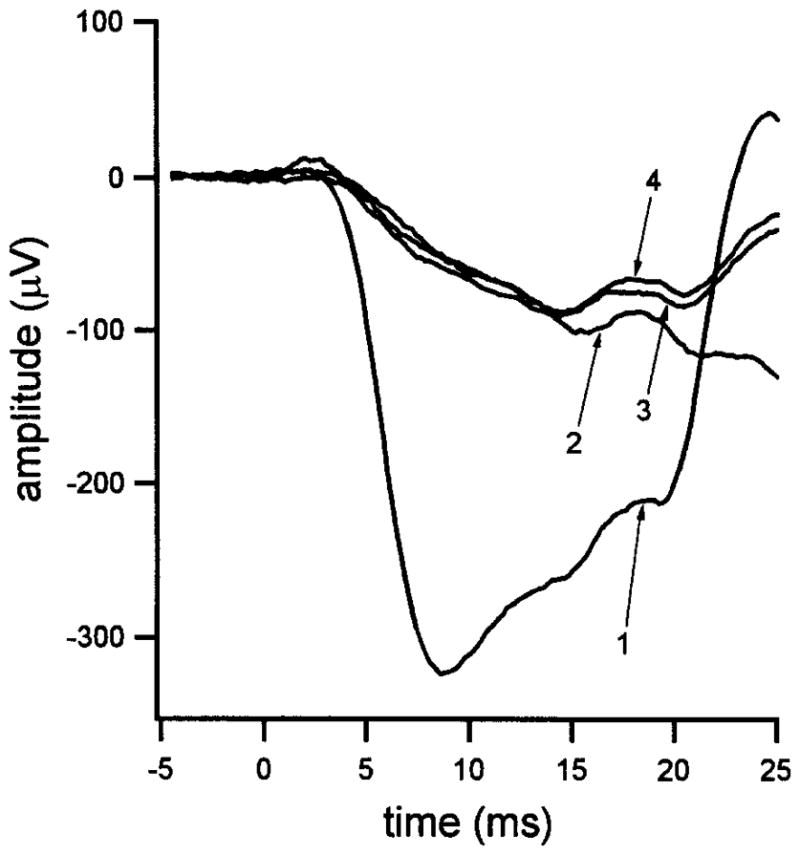
Responses to photopically matched short-wavelength (blue) and long-wavelength (red) flashes of high intensity presented at time zero. Trace 1: Response to the blue flash. The flash strength (1.6 × 104 sc td-sec) is representative of that of the blue probe flash used in paired-flash experiments. Trace 2: Response to the photopically matched red flash. Trace 3: Response to the blue flash presented 3 sec after a brilliant conditioning flash (3.4 × 105 sc td-sec). Trace 4: Response to the photopically matched red flash presented 3 sec after the same conditioning flash. Data replotted from Fig. 2 of Pepperberg et al.24, with permission of Cambridge University Press.
b-Wave and Other Postreceptor Components
Onset of the b-wave, together with oscillatory potentials and other postreceptor potentials, shapes the peak of the a-wave response in a manner dependent on both the test flash strength and the state of adaptation. The effects of these components on paired-flash determinations can be illustrated by considering how they might influence the kinetics of probe flash responses obtained with differing test flash strengths and a fixed interflash interval. Here, increasing the test flash strength can be expected to alter and ultimately saturate the b-wave of the test-flash-generated ERG (see, e.g., Hood and Birch12,32). This must have some nonzero effect on the response to the bright probe flash and, thus, on the derived amplitude of the test flash response. Experimental evidence indicates, however, that this effect is small for the case of weak test flashes. For example, rod-mediated probe responses obtained with a fixed interflash interval of 170 msec (i.e., obtained at a post-test-flash time near the peak of the derived rod response) exhibit generally similar normalized kinetics over their rising and near-peak phases. That is, these probe responses are roughly scaled versions of one another (Fig. 6 of Pepperberg et al.24). An approximate kinetic similarity is evident also among rod-mediated probe responses obtained with a fixed, relatively weak test flash and differing interflash intervals (Fig. 2C of Pepperberg et al.24). These findings argue against a large effect of altered b-wave kinetics on determination of the probe response amplitude and thus on the derived response to the test flash.
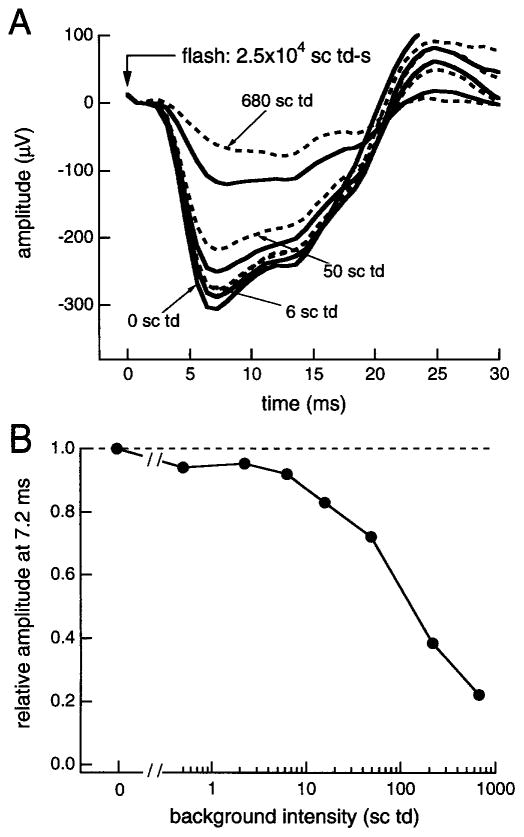
In addition to the cornea-positive b-wave, components of the same (cornea-negative) polarity as the photoreceptor response may contribute to the rod ERG generated by the probe flash.13,33–35 A test for the contribution of postreceptor, negative ERG components is to examine how background light affects the response to a flash of fixed high intensity, i.e., a flash comparable to the probe flash used in paired-flash experiments. It is known, for example, that the scotopic threshold response (STR) is adapted out, i.e., the flash-generated STR is eliminated, by backgrounds much weaker than those that significantly reduce the rod circulating current.35,36 Thus, if a postreceptor response contributed substantially to the a-wave produced by the bright flash under dark-adapted conditions (i.e., in the absence of background light), a decrease in the a-wave response from the maximum, fully dark-adapted level should be evident with relatively weak backgrounds. Figure 3 shows results obtained when the response to a fixed-intensity flash (2.5 × 104 sc td-sec) was measured in the presence of differing backgrounds (0–680 sc td).18 A substantial decrease from the dark-adapted amplitude (decrease from the dashed horizontal line in Fig. 3B) is apparent with backgrounds of ~50 sc td and higher. On the basis of in vitro photocurrent data from rods19–21 and estimates of the in vivo photoisomerizing strength of a given stimulus,10,37 this decrease is attributable to a reduction of the photocurrent excursion, i.e., to a decrease in the rod response itself. However, backgrounds below ~6 sc td produce only a small reduction from the dark-adapted amplitude. The data of Fig. 3 are consistent with those obtained from monkey in a similar type of experiment28 and in experiments involving pharmacological isolation of the photoreceptor response.38 Together, the results suggest that postreceptor negative components do not substantially skew determinations of the probe flash response in the present method, where the a-wave amplitude is measured shortly (<10 msec) after the probe flash.
Fig. 3.
Effect of background light (0–680 sc td) on the response to a flash of fixed high intensity (2.5 × 104 sc td-sec). (A) Flash responses obtained with differing backgrounds. (B) Relative amplitudes of the flash response determined 7.2 msec after flash presentation.
Desensitization of Rod-Mediated Probe Response
A further possible source of error in the paired-flash method relates to the assumption that the probe-driven saturation of the rods proceeds in kinetically invariant fashion. That is, the normalized rate at which the probe flash blocks the rod circulating current is presumed to be essentially constant. This assumption would no longer be valid if, for example, the rods were desensitized by steady background light or by the test flash being used in the experiment. Such desensitization would lead to a skewed determination of the derived response to the test flash. We have tested this possibility for the case of recovery from a near-saturating test flash, by investigating the dependence of the derived response amplitude at a fixed interflash interval (t = 500 msec) on the strength of the probe flash (Fig. 5B of Pepperberg et al.24). Varying the probe flash strength over an approximately 10-fold range (2.5 × 103–2.4 × 104 sc td-sec) was found to have little effect on the probe response amplitude and, thus, on the amplitude of the derived response to the test flash. A hint of desensitization is apparent shortly after extremely intense test flashes, in the somewhat reduced rate of rise of the probe flash response (e.g., Figs. 2 and 5 of Birch et al.,22 and present Fig. 3). However, such a change in kinetics is not conclusive evidence of rod desensitization, as the b-wave contribution to the probe flash response can also be substantially altered by a bright test flash.29
Time Course of Derived Response
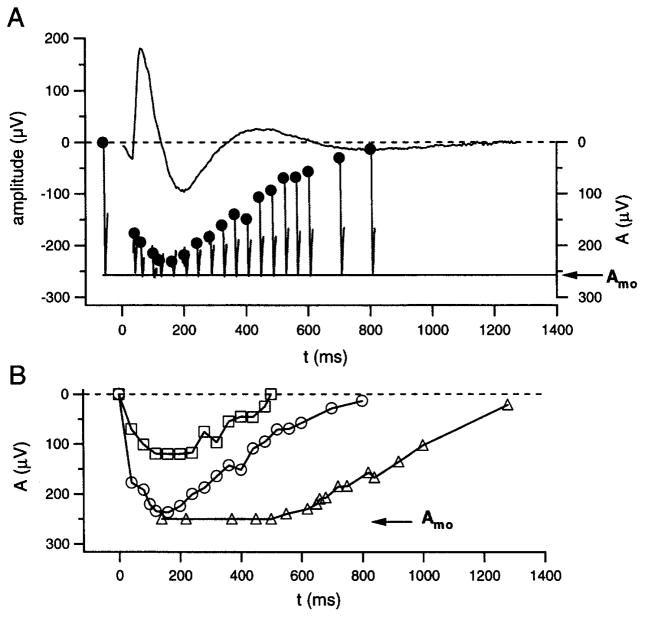
Figure 4 illustrates derived responses obtained by the use of the simple cone subtraction procedure described above, i.e., that in which the cone component of the probe flash response is taken as constant. The experiment of Fig. 4A involved presentation of a 44-sc td-sec test flash and a 1.2 × 104 sc td-sec blue probe flash in each of a series of paired-flash trials, and computational subtraction, from each of the raw responses to the blue probe, of the cone-mediated response to the photopically matched red probe. The rod-mediated probe responses resulting from this procedure, including that obtained for the probe-alone response (trace at the left), are positioned with their peaks at the assumed fixed level associated with photocurrent saturation (cf. Fig. 1). The pre-probe-flash baseline values determined from these responses (filled circles), as referred to the value obtained from the probe-alone response, represent the derived rod response to the test flash. The waveform in the upper part of Fig. 4A is the ERG response to the test flash delivered alone and is shown for comparison with the paired-flash results.
Fig. 4.
Derived rod response to a fixed-intensity flash. (A) Test flash of 44 sc td-sec. Hooklike traces are rod-mediated probe responses obtained in paired-flash trials with variation of the interflash interval t. Trace to the left of t = 0 is the probe-alone response. The response to a photopically matched red probe flash (not shown) was subtracted from the raw probe responses to yield the illustrated rod-mediated components. Filled circles represent baselines from which the probe responses depart. Presentation of the test flash alone yielded the ERG response illustrated at the top. (B) Derived rod responses to test flashes of 11 sc td-sec (squares), 44 sc td-sec [circles; same experiment as that of (A)], and 320 sc td-sec (triangles). Data obtained from a single subject. Results shown by squares and circles are replotted from Fig. 5 of Pepperberg et al.24, with permission of Cambridge University Press.
In Fig. 4B the derived response to the 44-sc td-sec test flash (circles) is shown together with the results of similar experiments that employed test flashes of 11 and 320 sc td-sec (squares and triangles, respectively). The derived response increases in peak amplitude and becomes longer in duration with increasing test flash strength, and the kinetics of the response are generally similar to those of photocurrent flash responses recorded from human rods in vitro.21,24 Analyses of the apparent saturation period and the subsequent recovery phase that characterize the derived response to a relatively bright test flash (e.g., triangles in Fig. 4B) have been conducted in both human subjects and in mice, with test flashes that extend into the range of significant rhodopsin bleaching.22,23,26,27,39
As noted above, the Fig. 4 experiments employed subtraction of a single response, one representing a cone contribution assumed constant under the investigated conditions, to isolate the rod-mediated component of the probe flash responses. A technically more complex but conceptually similar procedure can be used to determine the rod-mediated probe response under conditions associated with a rapidly changing cone component, e.g., those prevailing at early times (short interflash interval) after a moderate to bright test flash. Two related factors must be considered under conditions of this type. First, the cone photoreceptor response itself is expected to vary significantly with small changes in t, the interflash interval. Second, the b-wave and other postreceptor components of the cone-driven response may produce time-varying (i.e., t-dependent) changes in b-wave intrusion and, thus, variation in the shape of the probe response.
Figure 5 describes the use of this modified procedure to obtain derived amplitudes of the rod response at a fixed early time after a test flash. Responses PAb and PAr in Fig. 5A are probe-alone responses obtained, respectively, with the blue and photopically matched red probe flashes used in the illustrated experiment. As in the experiments described above, the difference between these two probe-alone responses (here, evaluated 6.4 msec after the probe flash) was taken as Amo, the maximal excursion of the rod circulating current (vertical double-headed arrow in Fig. 5A). Also as in the Fig. 4 experiments, that of Fig. 5 involved paired-flash trials conducted with a blue test flash and, at a fixed later time (here 7.4 msec), a blue probe flash. Here, however, at each of the test flash strengths investigated, we also conducted a paired-flash trial that employed the identical test flash (blue) and interflash interval (7.4 msec) but used a photopically matched red probe flash rather than the blue probe flash. Records TPb (test flash and blue probe flash) and TPr (test flash and red probe flash) in Fig. 5B show the paired-flash ERG responses obtained, respectively, with the blue and red probe flashes, and with the blue test flash strength in both trials set at 174 sc td-sec. By analogy with the cone subtraction procedure used in the Fig. 4 experiments, the difference between responses TPb and TPr determined 6.4 msec after probe flash presentation (vertical double-headed arrow in Fig. 5B) is taken as the rod-mediated probe response amplitude Am at the post-test-flash time of 13.8 msec (7.4–msec interflash interval plus 6.4 msec). With Eq. (1), the measured values of Amo and Am (Fig. 5A and B) yield determination of the normalized derived response A/Amo 13.8 msec after the 174-sc td-sec test flash.
Figure 5C illustrates an evaluation of the cone subtraction procedure just described. Waveforms in Fig. 5C represent rod-mediated a-wave responses to a series of test flashes, i.e., those obtained on correction for the cone contribution to the test-alone response. The dashed curve indicates the predicted response to a test flash of 3.5 × 103 sc td-sec; this curve was obtained by fitting (ensemble fit) a computational model of the a-wave leading edge to the family of waveforms.9,40 Open circles positioned at the post-test-flash time of 13.8 msec indicate paired-flash determinations of A/Amo, the normalized derived rod response, at four test flash strengths (those for which the a-wave peak occurred later than 13.8 msec) including the 174-sc td-sec case described in Fig. 5B. These A/Amo data have been scaled so as to equate the maximal excursion determined from the a-wave data (asymptote of the dashed curve) with Amo, the maximal excursion determined 6.4 msec after the probe flash (Fig. 5A). The correspondence of the paired-flash results (circles) with the a-wave responses provides evidence of the validity of the procedure used here to derive the rod-mediated response to a relatively strong test flash at short post-test-flash times.
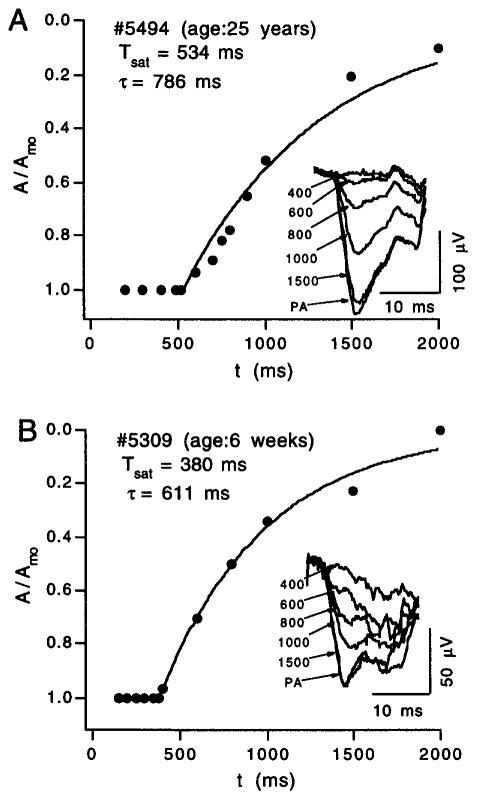
The derived rod response to a test flash within the range of ~102–106 sc td-sec is characterized by a period (Tsat) of near-complete saturation (i.e., of near-complete suppression of the rod circulating current) and by a subsequent, approximately exponential recovery phase.22,23 As shown by the Fig. 6A results obtained from an adult subject, fitting an exponential function to the paired-flash data yields determinations of the saturation period Tsat and of τ, an exponential time constant describing recovery (see caption to Fig. 6). Because the paired-flash method involves rapid titration of the circulating current, i.e., development of the probe-flash-generated a-wave response within about 8 msec, the derived response obtained with the method is relatively insensitive to low-frequency noise and baseline drift. It is thus possible, for example, to study the kinetics of rod recovery even in infant subjects. Figure 6B shows results obtained from an infant of age 6 weeks. Despite amplitudes that are considerably smaller than those obtained from the adult subject under similar stimulus conditions (Fig. 6A), the time courses of recovery are comparable. In contrast to the maturation evident for the activation stages of rod phototransduction,31,41 the data of Fig. 6 suggest that processes underlying recovery of the rod response may be essentially fully developed at or soon after birth.
Fig. 6.
Rod recovery time courses obtained from an adult (A) and from an infant of age 6 weeks (B). Data within each panel plot the relative amplitude A/Amo of the derived rod response to a fixed-intensity test flash in relation to the interflash interval t. Test flash strengths in the two experiments were comparable (200–300 sc td-sec). The smooth curve fitted to each set of data plots the relation, A/Amo = exp[−(t − Tsat)/τ], where Tsat is the period of apparent rod saturation that precedes recovery and τ is a recovery time constant. The inset of each panel shows representative rod-mediated probe responses; labels identify the interflash interval, in milliseconds. Response PA is the probe-alone response.
Amplitude–Intensity Relation
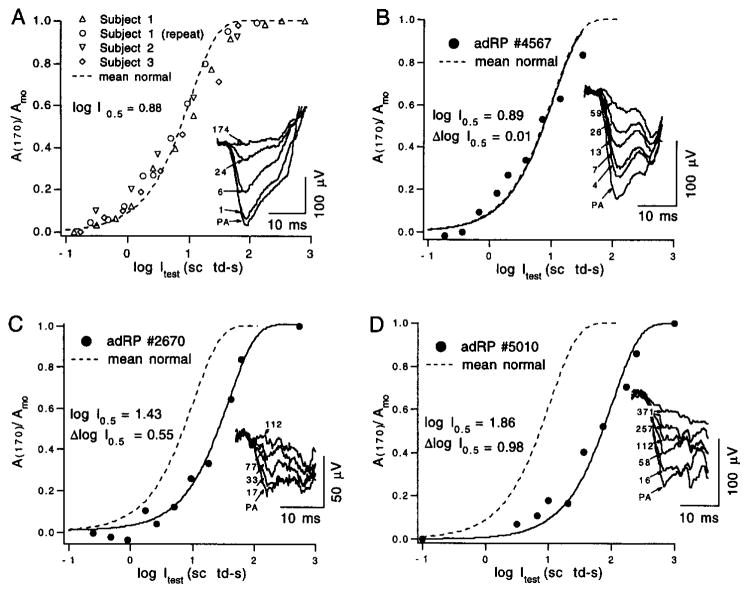
To determine the amplitude–intensity relation (response function) of the derived rod response, the interflash interval is held constant, and the derived amplitude is examined in relation to the strength of the test flash. Figure 7A shows results of determinations in normal subjects at an interflash interval of 170 msec, i.e., near the peak of the derived response. The smooth curve is an exponential saturation function19,42 with half-saturation at a flash strength (I0.5) of 7.6 sc td-sec (see caption to Fig. 7). The generally good fit of this curve to the data is consistent with the correspondence of this type of function with in vitro photocurrent data. Determining the photoisomerizing strength of the test flash at a given point on the amplitude–intensity relation, e.g., at half-saturation of the response, requires knowledge of the number of rhodopsins per rod isomerized (activated) by a flash of unit strength, i.e., by a flash strength of 1 scotopic troland-second (sc td-sec). This conversion factor is based on a number of estimates such as those for preretinal absorption, the size and packing of the rods, and the quantum efficiency of the rod. Estimates for the human eye indicate that 1 sc td-sec is equivalent, on average, to between 4.3 and 8.6 photoisomerizations per rod.10,37 As the half-saturation flash strength I0.5 is about 7.6 sc td-sec in normal subjects (Fig. 7A), use of these estimates yields the range of about 33 to 66 photoactivated rhodopsins per rod, on average, at half-saturation of the near-peak (170 msec) amplitude of the human rod flash response.
Fig. 7.
Amplitude–intensity functions for the derived rod response obtained with a fixed interflash interval (170 msec). (A) Data collected from three normal subjects (see legend). The dashed curve plots the saturating exponential relation, A(170)/Amo = 1 − exp[−(ln 2) Ites,/I0.5], where Itest is the test flash strength and I0.5 is the test flash strength at half-saturation. For the curve in (A), I0.5 = 7.6 sc td-sec (i.e., log I0.5 = 0.88). Inset: Rod-mediated probe responses obtained with test flashes of the indicated strength, in scotopic troland-seconds. Response PA is the probe-alone response. Data replotted from Fig. 6 of Pepperberg et al.24 with permission of Cambridge University Press. (B–D) Results obtained from three patients with autosomal dominant retinitis pigmentosa. The solid curves in (B–D) describe the fit of the preceding exponential relation to the data obtained from the patients. The dashed curves replot the curve of (A) (data from normal subjects). Values of log I0.5 and of Δlog I0.5, the logarithmic shift of the exponential relation relative to that of (A), are shown. Insets show representative rod-mediated probe responses. See text for further details.
Clinical Applications
Properties of the response derived from paired-flash measurements provide insight into photoreceptor abnormalities in diseases such as retinitis pigmentosa, and extend the analysis of phenotype beyond that possible by conventional ERG characterization. Figure 7B–D shows amplitude–intensity data obtained from three patients with autosomal dominant retinitis pigmentosa (adRP). As in Fig. 7A (results from normal subjects), an exponential saturation function (see caption to Fig. 7) has been fitted to each set of data to yield values for the half-saturation parameter I0.5 and, thus, for the relative position of the function along the axis representing the logarithm of the test flash strength (log Itest). Functions from these three patients reflect the wide variation in sensitivity loss (Δlog I0.5 in Fig. 7B–D) evident even among patients of similar age. Patient 4567 (Fig. 7B) has a sector form of adRP with field loss limited to the superior far periphery. She does not report night blindness, and final dark-adapted rod threshold determined psychophysically is normal at 7° eccentricity. Patient 2670 (Fig. 7C) has a form of adRP caused by a point mutation in codon 185 of the rhodopsin gene (Cys185Arg). His visual field is constricted to 25° in diameter, but within the central field he retains considerable rod function with an elevation of only 0.5 log unit in dark-adapted rod threshold. Patient 5010 (Fig. 7D) has a Pro23His rhodopsin mutation. Although his field is larger than that of patient 2670 and his rod-mediated probe flash responses are larger (compare responses shown in the insets of Fig. 7C and D), his amplitude–intensity relation exhibits a desensitizing shift considerably greater than that of patient 2670 (compare values of Δlog I0.5 in Fig. 7C and D).
Summary and Areas for Future Investigation
Studies to date investigating the paired-flash technique in human subjects indicate that this method yields, to good approximation, the full time course of the massed rod photoreceptor response to a test flash.22–24 Support for this conclusion comes primarily from the correspondence of kinetic, sensitivity, and light adaptation properties of the paired-flash-derived response with photocurrent response properties of human and other mammalian rods in vitro.19–21 Results obtained with the paired-flash method motivate further work in both human subjects and experimental animals to refine this in vivo approach for studying rod phototransduction. The present chapter has identified a number of considerations likely to be important for future development of the technique, including the refinement of procedures to subtract the cone contribution at short interflash intervals, and to better correct for postreceptor contributions and desensitization of the probe flash response. Improvements and extensions of the paired-flash technique should prove valuable for both fundamental and clinically oriented studies of the rod photoreceptor response.
A related and particularly interesting avenue for future work is paired-flash determination of the in vivo response properties of cone photoreceptors. Some information of this type obtained from human subjects has been reported.43,44 In these experiments, contributions of the rods to the measured responses have been suppressed by the use of intense, i.e., rod-saturating, backgrounds. As in the case of the paired-flash-derived response of the rods, that determined for cones43 shows quantitative agreement with single-cell data.45 Thus far, however, relatively few conditions of background and test flash strength have been investigated, and much remains to be done with regard to determining the contributions of different cone types to the derived response.
Acknowledgments
We thank Dr. J. R. Hetling for helpful comments on the manuscript. Research in our laboratories is supported by Grants EY-05494, EY-05235, EY-09076, and HD-22380 from the National Institutes of Health; by Research to Prevent Blindness, Inc. (New York, NY); and by The Foundation Fighting Blindness (Baltimore, MD).
References
- 1.Granit R. J Physiol. 1933;77:207. doi: 10.1113/jphysiol.1933.sp002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn RD, Hagins WA. Nature (London) 1969;223:201. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- 3.Heynen H, van Norren D. Vision Res. 1985;25:697. doi: 10.1016/0042-6989(85)90176-2. [DOI] [PubMed] [Google Scholar]
- 4.Heynen H, van Norren D. Vision Res. 1985;25:709. doi: 10.1016/0042-6989(85)90177-4. [DOI] [PubMed] [Google Scholar]
- 5.Hood DC, Birch DG. Visual Neurosci. 1990;5:379. doi: 10.1017/s0952523800000468. [DOI] [PubMed] [Google Scholar]
- 6.Hood DC, Birch DG. Invest Ophthalmol Vis Sci. 1990;31:2070. [PubMed] [Google Scholar]
- 7.Breton ME, Montzka DP. Doc Ophthalmol. 1992;79:337. doi: 10.1007/BF00160948. [DOI] [PubMed] [Google Scholar]
- 8.Cideciyan AV, Jacobson SG. Invest Ophthalmol Vis Sci. 1993;34:3253. [PubMed] [Google Scholar]
- 9.Hood DC, Birch DG. Vision Res. 1993;33:1605. doi: 10.1016/0042-6989(93)90027-t. [DOI] [PubMed] [Google Scholar]
- 10.Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Invest Ophthalmol Vis Sci. 1994;35:295. [PubMed] [Google Scholar]
- 11.Robson JG, Frishman LJ. Visual Neurosci. 1995;12:837. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- 12.Hood DC, Birch DG. Visual Neurosci. 1992;8:107. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- 13.Robson JG, Frishman LJ. J Opt Soc Am A. 1996;13:613. doi: 10.1364/josaa.13.000613. [DOI] [PubMed] [Google Scholar]
- 14.Wachtmeister L. Prog Ret Eye Res. 1998;17:485. doi: 10.1016/s1350-9462(98)00006-8. [DOI] [PubMed] [Google Scholar]
- 15.Hood DC, Birch DG. Invest Ophthalmol Vis Sci. 1994;35:2948. [PubMed] [Google Scholar]
- 16.Cideciyan AV, Jacobson SG. Vision Res. 1996;36:2609. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 17.Birch DG, Hood DC. In: Degenerative Diseases of the Retina. Anderson R, Hollyfield J, LaVail M, editors. Plenum; New York: 1995. p. 359. [Google Scholar]
- 18.Hood DC, Birch DG. Doc Ophthalmol. 1997;92:253. doi: 10.1007/BF02584080. [DOI] [PubMed] [Google Scholar]
- 19.Baylor DA, Nunn BJ, Schnapf JL. J Physiol. 1984;357:575. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakatani K, Tamura T, Yau KW. J Gen Physiol. 1991;97:413. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft TW, Schneeweis DM, Schnapf JL. J Physiol. 1993;464:747. doi: 10.1113/jphysiol.1993.sp019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Invest Ophthalmol Vis Sci. 1995;36:1603. [PubMed] [Google Scholar]
- 23.Pepperberg DR, Birch DG, Hofmann KP, Hood DC. J Opt Soc Am A. 1996;13:586. doi: 10.1364/josaa.13.000586. [DOI] [PubMed] [Google Scholar]
- 24.Pepperberg DR, Birch DG, Hood DC. Visual Neurosci. 1997;14:73. doi: 10.1017/s0952523800008774. [DOI] [PubMed] [Google Scholar]
- 25.Birch DG, Kedzierski W, Nusinowitz S, Anderson JL, Travis GH. Invest Ophthalmol Vis Sci. 1995;36:S641. [Google Scholar]
- 26.Lyubarsky AL, Pugh EN., Jr J Neurosci. 1996;16:563. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto Y, Peachey NS, Ziroli NE, Seiple WH, Gryczan C, Pepperberg DR, Naash MI. J Opt Soc Am A. 1996;13:577. doi: 10.1364/josaa.13.000577. [DOI] [PubMed] [Google Scholar]
- 28.Robson JG, Frishman LJ, Viswanathan S. Invest Ophthalmol Vis Sci. 1997;38:S886. [PubMed] [Google Scholar]
- 29.Hetling JR, Pepperberg DR. J Physiol. 1999;516:593. doi: 10.1111/j.1469-7793.1999.0593v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yau KW. Invest Ophthalmol Vis Sci. 1994;35:9. [PubMed] [Google Scholar]
- 31.Nusinowitz S, Birch DG, Birch EE. Vision Res. 1998;38:627. doi: 10.1016/s0042-6989(97)00286-1. [DOI] [PubMed] [Google Scholar]
- 32.Hood DC, Birch DG. J Opt Soc Am A. 1996;13:623. doi: 10.1364/josaa.13.000623. [DOI] [PubMed] [Google Scholar]
- 33.Sillman AJ, Ito H, Tomita T. Vision Res. 1969;9:1435. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg RH, Frishman LJ, Sieving PA. Prog Ret Res. 1991;10:121. [Google Scholar]
- 35.Frishman LJ, Reddy MG, Robson JG. J Opt Soc Am A. 1996;13:601. doi: 10.1364/josaa.13.000601. [DOI] [PubMed] [Google Scholar]
- 36.Frishman LJ, Sieving PA. Vision Res. 1995;35:435. doi: 10.1016/0042-6989(94)00165-i. [DOI] [PubMed] [Google Scholar]
- 37.Makous WL. J Opt Soc Am A. 1997;14:2323. doi: 10.1364/josaa.14.002323. [DOI] [PubMed] [Google Scholar]
- 38.Jamison JA, Bush RA, Sieving PA. Invest Ophthalmol Vis Sci. 1996;37:S136. [PubMed] [Google Scholar]
- 39.Thomas MM, Lamb TD. J Physiol. 1999;518:479. doi: 10.1111/j.1469-7793.1999.0479p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb TD, Pugh EN., Jr J Physiol. 1992;449:719. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulton AB, Hansen RM. Curr Eye Res. 1992;11:1193. doi: 10.3109/02713689208999544. [DOI] [PubMed] [Google Scholar]
- 42.Lamb TD, McNaughton PA, Yau KW. J Physiol. 1981;319:463. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood DC, Birch DG, Pepperberg DR. 1996 Optical Soc Amer Technical Digest Series. Vol. 1. Optical Society of America; Washington, D.C: Vision Science and Its Applications; p. 64. [Google Scholar]
- 44.Cideciyan AV, Zhao X, Nielsen L, Khani SC, Jacobson SG, Palczewski K. Proc Natl Acad Sci USA. 1998;95:328. doi: 10.1073/pnas.95.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnapf JL, Nunn BJ, Meister M, Baylor DA. J Physiol. 1990;427:681. doi: 10.1113/jphysiol.1990.sp018193. [DOI] [PMC free article] [PubMed] [Google Scholar]