Fig. 9.
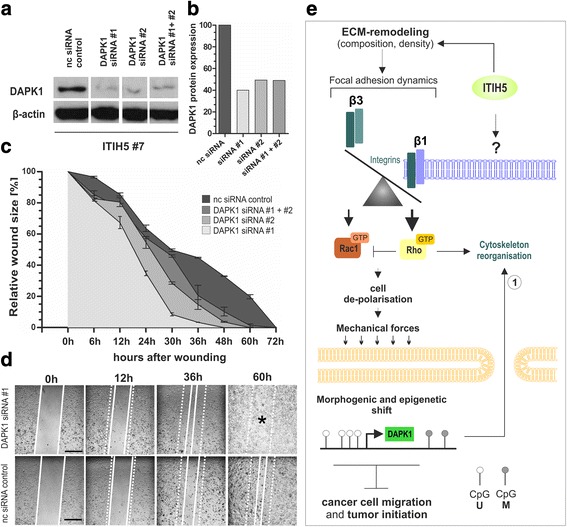
DAPK1 knockdown restore a motile phenotype in ΔpBK-ITIH5 cells in vitro. a DAPK1 protein expression in ITIH5-expressing cells 48 h after transfection with DAPK1-siRNA #1, DAPK1-siRNA #2 as well as #1 and #2 combined in comparison to nc siRNA transfected control cells. β-actin served as loading control. b Densitometric determination of DAPK1 protein knockdown in ΔpBK-ITIH5 cells compared to control. c Cell migration of ITIH5 clones after treatment with DAPK1-siRNAs was analyzed by using a wound healing assay over 72 h. nc siRNA transfected cells served as negative control. Vertical lines: standard deviation (S.D.). Cell-free area on day 0 was set as 100% and used for standardization. d Representative wound area documentation by light microscopy of DAPK1-siRNA #1 and nc siRNA control 0, 12, 36, and 60 h after scratching. White line: cell-free wound area. White dashed line: original wound area size at 0 h. Scale bar: 500 μm. e Working model highlighting factors potentially involved in ITIH5-driven phenotype shift of mesenchymal MDA-MB-231 breast cancer cells towards an epithelial-like state. ITIH5 remodels the ECM that is accompanied by changes in integrin composition. As a consequence downstream signaling is shifted towards RhoA activation. Clustered cancer cells further lacked polarization but featured in turn strong cell-matrix adhesion and modulated biomechanical cues. Re-expression of DAPK1, caused by epigenetic reprogramming, may be finally involved in ITIH5 mediated suppression of tumor cell migration dynamics potentially by re-organization of cytoskeleton structures as recently described (1): [43, 44]
