Figure 1.
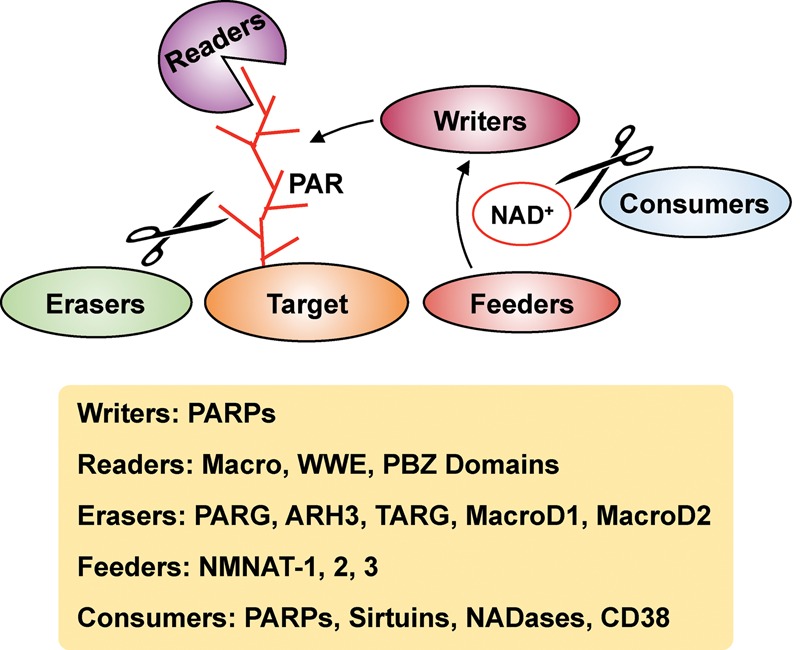
A variety of effectors mediate intracellular ADP-ribosylation dynamics. PARPs act as “writers” that add ADP-ribose moieties to target proteins. The NAD+ required for these PARP-mediated ADP-ribosylation reactions is supplied by nicotinamide mononucleotide adenylyl transferases (NMNATs; “feeders”). The ADP-ribose units on target protein can be recognized by “readers” containing macro, WWE, or PAR-binding zinc finger (PBZ) domains. The removal of ADP-ribose chains is catalyzed by “erasers,” which include PAR glycohydrolase (PARG), ADP-ribosyl hydrolase 3 (ARH3), TARG, and MacroD1/D2. NAD+ levels can be modulated by NAD+ “consumers,” such as PARPs, sirtuins, NADases, and CD38, which hydrolyze NAD+.
