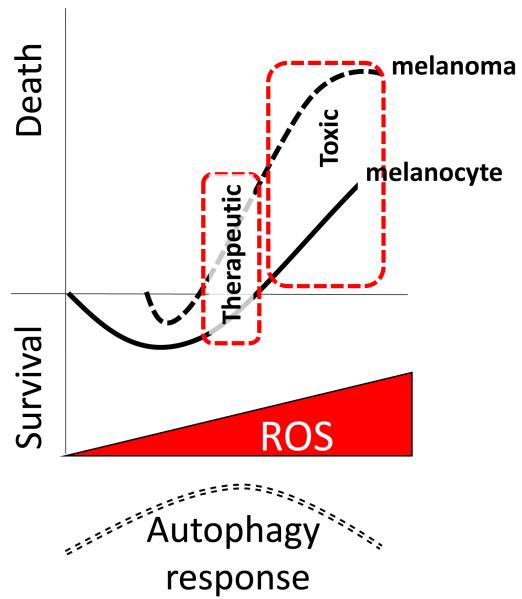
Figure 1. Hormetic effect of ROS increase on melanoma versus melanocyte cell fate: harnessing for therapeutic potential.
The proposed model is based on the threshold premise of reactive oxygen species having differential effects on cell response. The hormetic principle proposed here speculates that different levels of ROS are stimulatory for melanoma and melanocyte survival and provoke survival responses such as autophagy in attempt to rid the cell of oxidized/damaged contents. However, as ROS increases beyond the cell’s coping threshold, cell fate switches to death as apoptotic mechanisms ensue. The autophagy response (dashed curve, bottom) is induction as ROS increases, but eventual shut down/decline of the pro-survival autophagic mechanisms. We propose that the hormetic curve is altered in melanoma cells (dashed line) so that at the same amount of exogenous ROS generation, the melanocyte’s response (solid line) will be survival and the melanoma cell response will be death. This represents a therapeutic window for ROS-generating anti-cancer agents, and past a certain threshold, high enough ROS will lead to toxicity in both cell types. Contributing factors to this proposed hormetic alteration is based on literature showing decreased antioxidant capacity in melanoma cells owed to mutations in SOD1/2, and the existing basal oxidative stress state in melanoma cells that render them particularly susceptible to further ROS escalation.