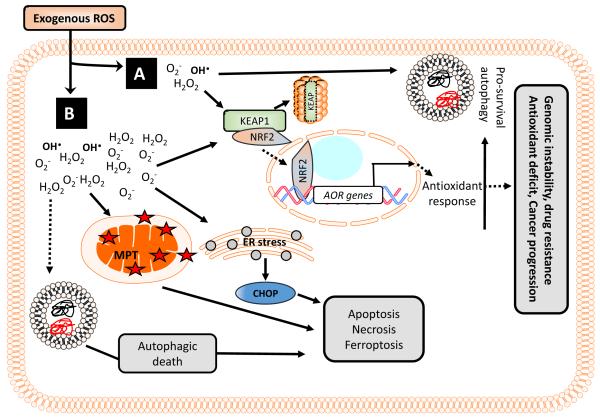
Figure 2. Role of ROS-mediated autophagy during melanoma cell fate determination.
(A) Cancer cells respond to low or transient exogenous ROS generation (chemotherapy, radiation) by KEAP1/NRF2-mediated oxidative stress response, and ER-mediated unfolded protein response to rid the cell of damaged lipids and proteins. Autophagy is initiated and sustained, leading to cell survival. The result is a cell with increased baseline autophagic activity, and continued exogenous ROS-induced stress leads to increased redox stress, genomic instability and therapeutic resistance over time. (B) Cancer cells respond to high or sustained exogenous ROS generation by an attempted KEAP1/NRF2-mediated oxidative stress response and initial autophagy activation. However, sustained ER stress, mitochondrial membrane depolarization, and activation of the caspase cascade results in the a pro-death response, by which time the autophagy response has been inhibited because the cell has committed to die. The molecular switch by which autophagy can no longer serve as a survival mechanism occurs when autophagy proteins become dysfunctional, participate in pro-apoptotic signaling, are post-translationally modified or the cell’s pro-apoptotic, necrotic, or ferroptotic signals have hierarchy above the proautophagic response. In some instances, a sustained autophagic response may result in autophagic cell death in the absence of pro-apoptotic signaling.