ABSTRACT
Background: Although recent studies have revealed TAR (trans-activating response region) DNA binding protein (TDP-43) as a potential therapeutic target for cancers, its role and clinical association with melanoma have not been explored. Objective: To identify the role and function of TDP-43 during melanoma pathogenesis. Methods: Firstly, the relationship between TDP-43 expression and patient survival was explored. Then TDP-43 expression level in melanoma tissue and different melanoma cell lines was measured. After silencing TDP-43 expression in melanoma cells, the impacts of TDP-43 on cellular proliferation, metastasis, glucose uptake, and glucose transporters levels were studied. In the end, effect of TDP-43 depletion on tumorigenicity of melanoma cells was tested in vivo. Results: Our results showed that TDP-43 was overexpressed in melanoma paraffin samples compared with that in nevi tissues. The high expression level of TDP-43 was associated with poor patient survival. By silencing TDP-43, we saw significant inhibition of cell proliferation and metastasis in A375 and WM451 cells. TDP-43 knockdown could suppress glucose transporter type-4 (GLUT4) expression and reduce glucose uptake. And downregulation of GLUT4 in melanoma cells induced inhibition of cell proliferation and metastasis. TDP-43 knockdown significantly slowed down tumor growth and decreased GLUT4 expression in vivo. Conclusion: TDP-43 is a novel oncogene in melanoma and regulates melanoma proliferation and metastasis potentially through modulation of glucose metabolism.
KEYWORDS: GLUT4, glucose metabolism, melanoma, metastasis, proliferation, TDP-43
Abbreviations
- TDP-43
TAR DNA binding protein
- GLUT
glucose transporter
- GLUT4
glucose transporter type-4
- HM
human melanocytes
Introduction
Melanoma is the most malignant superficial tumor, and is responsible for 80% of skin cancer related deaths. 1,2 Advanced melanoma gives a poor prognosis of a 5-year survival rate less than 15%.3,4 So, it is of great significance to explore its tumorigenicity mechanism, and to find out new bio-markers for early diagnosis, surveillance, and therapeutic targets.
TDP-43 is a RNA binding protein expressed in many tissues and encoded by TARDBP gene. 5 TDP-43 plays important roles in RNA maturation process. It also takes part in regulations of cell cycle and glucose/lipid metabolism. 6,7 Previous studies mostly focused on relations between abnormal TDP-43 expression and neurodegenerative diseases. 8 Meanwhile, recent studies showed a significant relation between TDP-43 and human cancer. TARDBP gene mutation contributes to Ewing sarcoma susceptibility. 9 In hepatocellular carcinoma, high TDP-43 expression regulates glycolysis level through TDP-43/miR-520/PFKP axis. 6 While in non-small-cell lung cancer (NSCLC) cells, TDP-43 downregulation suppresses proliferation, migration and invasion. 10 However the functional role of TDP-43 in melanoma progression and its association with clinical outcome is still unclear.
Glucose is the chief energy source of tumor cells. Increased glucose uptake plays an important role in tumor cells survival and proliferation. Glucose transporters (GLUTs), the key rate-limiting factors in glucose metabolism, were reported increased in various kinds of tumor cells and were strongly associated with proliferation and metastasis abilities of tumors, including melanoma. 11,12 A decreased expression of GLUT1 was found in TDP-43-depleted hepatocellular carcinoma cells, 6 indicating TDP-43 may be involved in the glucose uptake.
Here, we firstly reported that higher TDP-43 level was associated with poorer overall 5-year survival of melanoma patients. TDP-43 was significantly upreglulated in melanoma tissue and different melanoma cell lines. Functional analysis presented that TDP-43 silencing reduced proliferation and metastasis of melanoma cells. TDP-43 knockdown also downregulated GLUT4 expression with consequent decrease in glucose uptake. GLUT4 knockdown inhibited the proliferation and metastasis of melanoma cells. The in vivo study confirmed that TDP-43 knockdown inhibited tumorigenicity and GLUT4 expression. In conclusion, our results showed that TDP-43 is aberrantly expressed during the development of melanoma and may regulate melanoma cell proliferation and metastasis by modulation of glucose metabolism.
Results
Increased expression of TDP-43 was correlated with the poor survival of melanoma patients
To identify the role of TDP-43 in melanoma, immunohistochemistry analysis was performed for paraffin samples (128 cases of melanoma tissues and 66 cases of melanocytic nevi tissues, patient basic profiles were showed in Table 1) to evaluate the clinical value of TDP-43. Results showed that TDP-43 was expressed in 69.5% of melanoma tissue, which is significantly higher than 22.7% in melanocytic nevi tissue (Fig. 1A). Among the 128 cases of melanoma tissues, 30.47% were negative staining (−), 29.69% weak positive staining (+), 33.59% positive staining (++), and 6.25% strong positive staining (+++) (Fig. 1B). Representative immunohistochemical analysis of TDP-43 expression in melanoma tissues could been seen in Fig. 1C. To discern whether the TDP-43 expression was connected with the clinicopathological features, melanoma patients were seperated by age, gender, tumor location, stage and lymphatic metastasis. As showed in Table 2, melanoma tissues from stage III/IV and with lymph node metastasis had a higher TDP-43 expression, while TDP-43 expression showed no significant relation with age, gender, and tumor location. To further explore whether TDP-43 could be a prognostic factor for melanoma patient survival, Kaplan-Meier survival curves were drawn. The results showed that the overall 5 year-survival rate was significantly higher in low TDP-43 expression patients than that in high TDP-43 expression patients (Fig. 1D). Taken together, those results revealed an important role of TDP-43 in development of melanoma.
Table 1.
General characteristics of malignant melanoma and nevus group.
| Malignant melanoma | Nevus | |
|---|---|---|
| Total number of patients | 128 | 66 |
| Female/male* | 56/72 | 27/39 |
| Mean age** | 55.63 ± 13.43 | 27.73 ± 17.72 |
| Positivity/negative*** | 89/39 | 15/51 |
χ2 = 0.144, P = 0.705;
T = 8.407, P = 0.000;
χ2 = 38.357, P = 0.000
Figure 1.
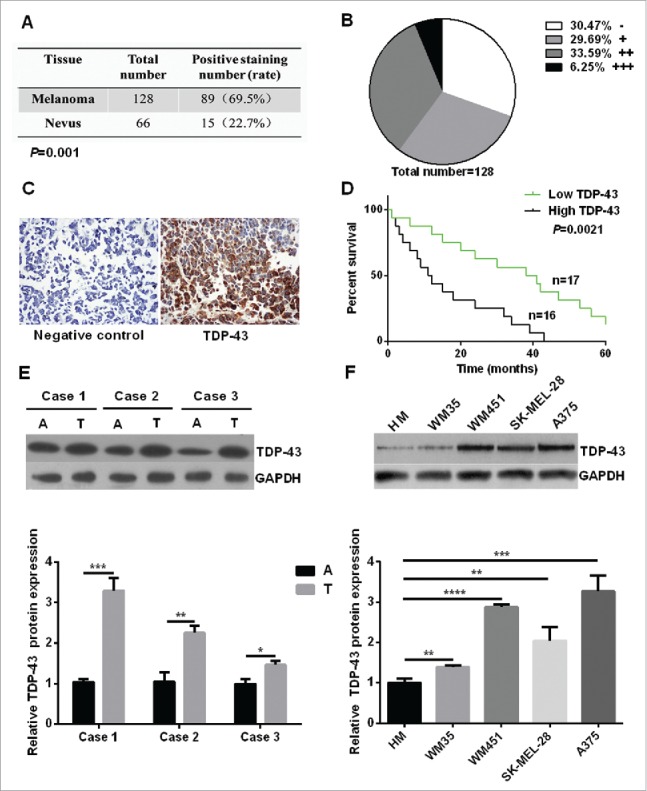
Increased expression of TDP-43 was correlated with the poor survival of melanoma patients. (A) Immunohistochemistry analysis showed that TDP-43 had a positive staining rate of 69.5% in melanoma tissue, 22.7% in melanocytic nevi tissue (P = 0.001). (B) Grade positivity by number of positive cells and staining intensity as follow: weak positive (+, <25 % positive in all cells), positive (++, 2550%– positive cells), and strong positive (+++,>50 % positive cells). Out of 128 cases of melanoma tissues, 30.47% were negative staining (−), 29.69% (+), 33.59% (++), and 6.25% (+++). (C) Representative immunohistochemical analysis of TDP-43 expression in melanoma tissue. On the left is negative control, and on the right shows anti-TDP-43 immunostaining strong positive (+++) mainly in cytoplasm. (D) Kaplan-Meier survival curves for groups based on TDP-43. Higher overall 5 year-survival rate was seen in low TDP-43 expression patients than in high TDP-43 expression patients. A positive staining rate <25 % was considered to be low expression, and positive staining rate ≧25% was considered to be high expression. (E) Western Blot showed higher TDP-43 expression in melanoma tissue than that in matching adjacent tissue. (F) Western Blot showed higher TDP-43 expression in melanoma cell lines than that in HM cells. A = adjacent normal tissue, T = tumor tissue.
Table 2.
Characteristics of malignant melanoma patients
| Characteristics | N | No. of positivity (%) |
|---|---|---|
| Gendera | ||
| Male | 72 | 51 (70.8%) |
| Female | 56 | 38 (67.9%) |
| Age b(year) | ||
| >65 | 37 | 25(67.6%) |
| ≤65 | 91 | 64 (70.3%) |
| Tumor locationc | ||
| Superficial tumor | 96 | 68(70.8%) |
| Non-superficial tumor | 32 | 21 (65.6%) |
| TNM staged | ||
| I/II | 55 | 33 (60.0%) |
| III/IV | 26 | 22 (84.6%) |
| Metastasis with lymph nodee | ||
| Yes | 25 | 21(84.0%) |
| No | 56 | 34 (60.7%) |
χ2 = 0.132, P = 0.717,
χ2 = 0.095, P = 0.758,
χ2 = 0.307, P = 0.579,
χ2 = 4.908, P = 0.027, and
χ2 = 4.300, P = 0.038. 81 patients with TNM stage information were included.
Next we compared TDP-43 expression levels in melanoma tissues and corresponding adjacent normal tissues. We found that TDP-43 protein and mRNA levels were both significantly higher in melanoma tissues than that in adjacent normal tissues (Fig. 1E and Fig. S1A). Furthermore we explored TDP-43 expression in primary human melanocytes (HM), melanoma cell lines of WM35, WM451, SK-MEL-28, and A375. Results showed a higher expression in all 4 melanoma cell lines compared with HM cells (Fig. 1F and Fig. S1B).
Downregulation of TDP-43 inhibited proliferation and metastasis of melanoma cells
To further explore the biological functions of TDP-43 in melanoma cells, we examined whether downregulation of TDP-43 could affect the proliferation and metastasis ability of melanoma cells. Because of a higher TDP-43 expression in A375 and WM451 cells, we chose these 2 cell lines for further experiments. In this study, TDP-43 shRNA-1 and TDP-43 shRNA-2 both down-regulated TDP-43 expression (Fig. 2A, and Fig. S2). Our results showed that decreased proliferation and colony formation was observed in TDP-43 silencing A375 and WM451 cells compared with the NC groups (Fig. 2A, B). Scratch and Transwell invasion assay showed that TDP-43 shRNA-1 and TDP-43 shRNA-2 could significantly inhibit migration and invasion of A375 and WM451 cells (Fig. 2C, D). These results suggest that TDP-43 participates in proliferation and metastasis regulation in melanoma cells.
Figure 2.
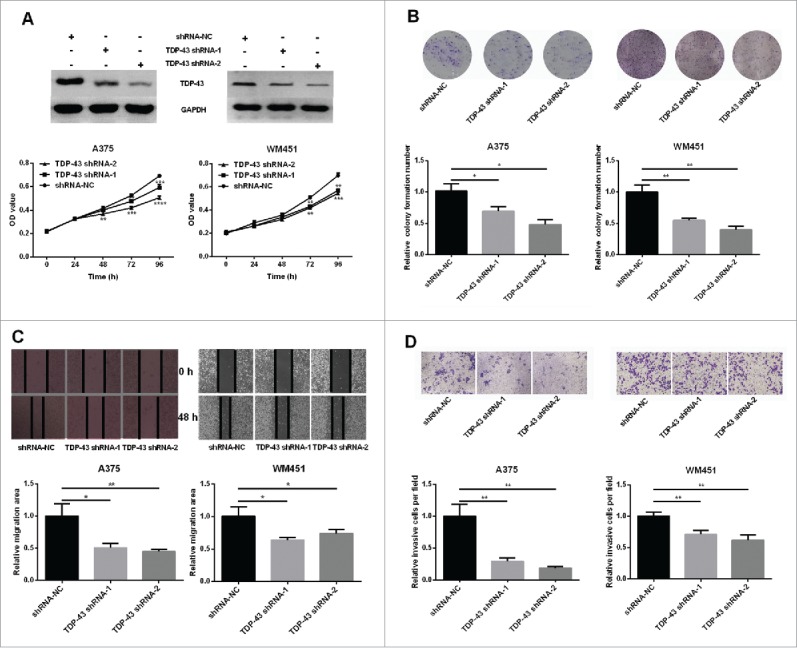
Involvement of TDP-43 in regulation of melanoma cell proliferation and metastasis (A-B) A375 and WM451 cells were transfected with shRNAs (shRNA-1 and shRNA-2) or negative control shRNA (NC) against TDP-43. Western Blot confirmed that both shRNA-1 and shRNA-2 could significantly reduce the TDP-43 expression. MTT assay showed decreased proliferative ability in TDP-43 shRNAs-transfected A375 and TDP-43 shRNAs-transfected WM451cells than that in NC cells. The colony formation efficiency was significantly lower in TDP-43 shRNAs-transfected A375 and TDP-43 shRNAs-transfected WM451cells than that in NC cells. (C-D) Scratch and Transwell invasion experiments showed inhibited migration and invasion ability in A375 and WM451 cells after down regulation of TDP-43. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
TDP-43 knockdown reduced glucose uptake and suppressed GLUT4 expression in melanoma cells
Considering that TDP-43 takes part in regulations of hepatocellular carcinoma glucose metabolism, 6 we wondered whether TDP-43 was involved in the regulation of glucose uptake in melanoma. The results showed that glucose uptake decreased in TDP-43 silencing A375 and WM451 cells compared with the NC cells (Fig. 3A). GLUT1, GLUT3, and GLUT4 are the most frequently studied glucose transporters to transfer glucose into cancer cells. Therefore we explored their mRNA expressions and found that TDP-43 shRNA-1 and TDP-43 shRNA-2 significantly suppressed GLUT4 mRNA expression, while no significant effect on GLUT1 and GLUT3 (Fig. 3B). Downregulation of GLUT4 protein was further confirmed in TDP-43 silencing A375 and WM451 cells by Western Blot (Fig. 3C). These data together indicate that decreased TDP-43 expression could suppress GLUT4 expression and reduce glucose uptake.
Figure 3.
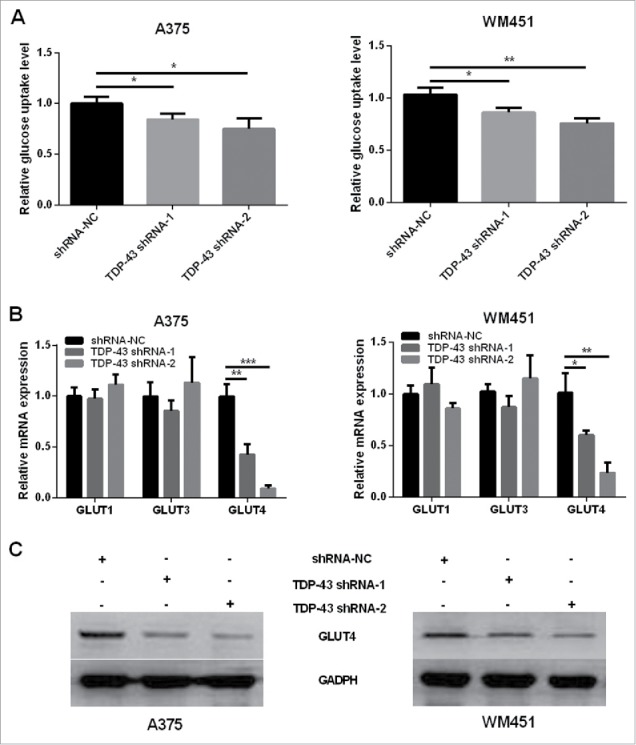
Knockdown of TDP-43 reduced glucose uptake and GLUT4 expression of melanoma cells. (A) Compared with NC cells, TDP-43 shRNAs (shRNA-1 and shRNA-2)-transfected A375 and TDP-43 shRNAs (shRNA-1 and shRNA-2)-transfected WM451 cells had decreased glucose uptake. (B) QRT-PCR showed that TDP-43 silencing A375 and WM451 cells had decreased GLUT4 mRNA expression, while GLUT1 and GLUT3 changes were not significant. (C) Western Blot confirmed the downregulation of GLUT4 in TDP-43 silencing A375 and WM451 cells. ***P<0.001, **P<0.01, *P <0.05.
Downregulation of GLUT4 inhibited proliferation and metastasis of melanoma cells
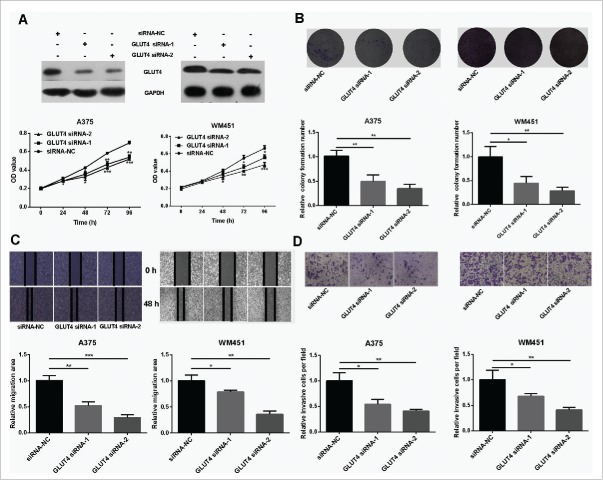
GLUT4 has been proved to participate in the development of cancers, such as breast cancer 13 and pancreatic cancer. 14 However, its role in melanoma is not well understood. To address this question, GLUT4 siRNAs were used to knockdown the expression of GLUT4 (Fig. 4A) and subsequently proliferation and metastasis abilities of melanoma cells were tested. The results showed that proliferation and metastasis abilities were dramatically reduced in GLUT4 knockdown A375 and WM451 cells (Fig. 4A, B, C, D). It is no coincidence that GLUT4 disruption impaired glucose uptake in A375 and WM451 cells (Fig. S3).
Figure 4.
Knockdown of GLUT4 reduces proliferation and metastasis of melanoma cells. (A) Western Blot confirmed that GLUT4 siRNA can significantly knockdown the expression of GLUT4. (B) MTT assay showed decreased proliferative ability in GLUT4 siRNA-transfected A375 and GLUT4 siRNA-transfected WM451 cells than that in NC cells. (C-D) Scratch and Transwell invasion experiments showed inhibited migration and invasion ability in A375 and WM451 cells after down regulation of GLUT4. ***P < 0.001, **P < 0.01, *P < 0.05.
Effect of TDP-43 depletion on tumorigenicity of melanoma cell lines
Finally, xenograft mouse model was used to validate the role of TDP-43 in vivo. The tumorigenicity experiment of melanoma cells was performed in A375 cell line because TDP-43 had the highest expression in A375 cells. TDP-43 shRNA-2 inhibited proliferation and metastasis of A375 more than TDP-43 shRNA-1 significantly. Therefore, TDP-43 shRNA-2-transfected A375 cells were used to implant into the subcutaneous tissue of nude mice. Nude mice tumorigenicity experiments showed that TDP-43 shRNA-2 significantly slowed down tumor growth (Fig. 5A, B, C, D). Western Blot of the tumor tissues showed decreased GLUT4 expression in TDP-43-knockdown melanoma tumors (Fig. 5E), indicating that TDP-43 depletion affects GLUT4 expression in vivo. TDP-43 expression in tumor tissues also showed decreased TDP-43 expression in TDP-43-knockdown melanoma tumors, compared with the NC group (Fig. S4). In addition, the in vitro effect of shRNA-1 targeting TDP-43 also significantly inhibited tumorigenicity (Fig. S5).
Figure 5.
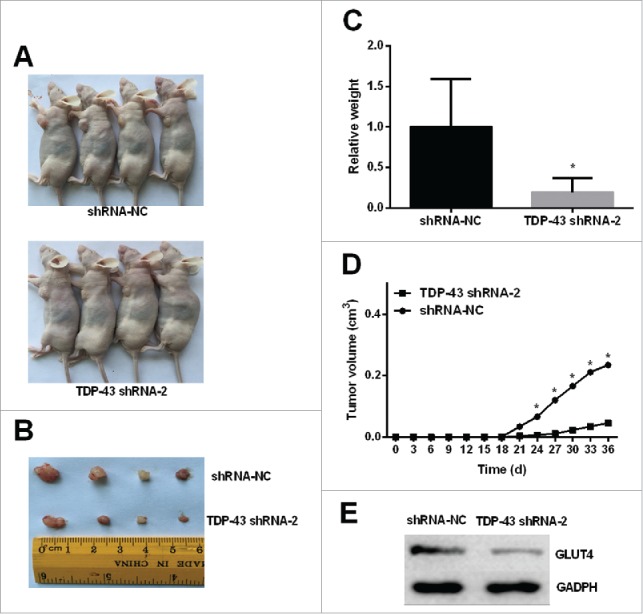
TDP-43 depletion affects tumorigenicity of A375 cell line and GLUT4 expression in vivo. Nonsilencing shRNA-transfected A375 cells and TDP-43 shRNA-2-transfected A375 cells with transfection efficiency greater than 80% were inoculated to right axillary fossa of each nude mice. (A-B) Tumor tissues isolated from indicated mice at day 35 post-transplant. (C-D) TDP-43 shRNA-2 significantly decreased the tumor weights and volumes. (E) Western Blot showed decreased GLUT4 expression in TDP-43-knockdown melanoma tumors. *P < 0.05.
Discussion
In this study, we firstly found that compared with 22.7% in melanocytic nevi sections, TDP-43 was expressed in 69.5% melanoma tissue sections, and an even higher expression rate in melanoma of stage III / IV or with lymph node metastasis. This result suggests that TDP-43 takes part in development of melanoma. In addition, higher TDP-43 expression associated with poorer melanoma patient survival indicates that TDP-43 is a potential molecular prognostic marker for melanoma.
Increased TDP-43 expression in melanoma tissues and melanoma cells was observed, suggesting its potential role as an oncogenic factor that faciliates the process of melanoma pathogenesis. In support of the hypothesis, we demonstrated that decreased expression of TDP-43 markedly led to decrease of melanoma cells proliferation and metastasis. In vivo, TDP-43 knockdown reduced the tumorigenicity of melanoma cells when orthotopically implanted into nude mice, which clearly demonstrates that TDP-43 plays a critical role in promoting melanoma process. These results are consistent with the oncogenic role of TDP-43 in hepatocellular carcinoma and in NSCLC. 6,10 Noteworthily, only mutant V600E BRAF cell lines were used in this study. TDP-43 expression in BRAF wild-type cell lines was not measured. Further studies are necessary to confirm if TDP-43 expression and V600E BRAF mutant are related.
Furthermore, after silencing TDP-43 expression in melanoma cells, we saw decreased GLUT4 expression and subsequently decreased glucose uptake. Glucose is the primary source of energy for cells. Abundant glucose uptake is important to tumor cells survival and growth. GLUT4 is one of the rate-limiting transporters for cells glucose uptake, and is found over-expressed in many cancers including myeloma, breast cancer, and melanoma.13,15,16 Hence GLUT4 was assumed to be invovled in the melanoma cells malignant behaviors. Decreased proliferation and metastasis abilities in GLUT4-deleted melanoma cells in our study confirmed the hypothesis. TDP-43 knockdown reduced the GLUT4 expression in vitro and in vivo, indicating that TDP-43 may modulate melanoma cells proliferation and metastasis by down-regulating GLUT4 expression with consequent decrease in glucose uptake. TDP-43 modulates translation of mRNA by binding an UG-rich sequence. 17 However GLUT4 mRNA sequences did not contain the UG repeats, suggesting that TDP-43 may regulate the expression of GLUT4 in an indirect way. However the exact regulation mechanism needs to be further explored.
Conclusion
In summary, we uncovered that TDP-43 is highly overexpressed in melanoma and increased expression of TDP-43 is correlated with the poor survival of melanoma patients. TDP-43 regulates melanoma proliferation and metastasis potentially through modulation of GLUT4 expression. Together, our study reveals that TDP-43 is a novel oncogene in melanoma.
Materials and methods
Tissue samples and cell lines
This study was approved by the Ethics Committee of Third Xiangya Hospital, Central South University (S 093) and was carried out in accordance with the approved guidelines. All patients provided written informed consent. All melanoma tumor tissue samples and adjacent tissues were collected in the Third Xiangya Hospital of Central South University. For primary human melanocytes (HM), melanoma cell lines WM35, WM451, SK-MEL-28, and A375 information, refer to our previous publication.18
QRT-PCR and western blot
Total RNA was extracted from tissue or cells and reversely transcribed to cDNA. QRT-PCR was used to amplify mRNAs of TDP-43, GLUT1, GLUT3, and GLUT4 genes. Threshold cycle (CT) values were used to calculate the fold change of the respective mRNAs. The fold change was determined relative to a control after normalizing to a housekeeping gene using 2− △△Ct. Primer sequences of above genes can be found in Supplementary Material Table 1. Tissue or cells were processed for protein extraction. Twenty μg total proteins were loaded per well and were separated by 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then proteins in the gel were transferred to nitrocellulose membranes. Membranes were blocked for 1.5 h. Anti-TDP-43 (Abnova, clone 2E2-D3, Taiwan) or anti-GLUT4 (Abcam, Cambridge, UK) was applied as first antibodies to incubate overnight. After PBST wash, goat anti-mouse IgG-HRP (Proteintech, Chicago, USA) was used as secondary antibodies to incubate for 40–60 mins. Protein level was evaluated after exposure. Anti-GAPDH (Proteintech, Chicago, USA) was used as internal control.
TDP-43 and GLUT4 gene silencing
Two different shRNAs (shRNA-1 and shRNA-2; purchased from GeneChem, Shanghai, China) were used to target the TDP-43 gene. A nonsilencing shRNA (NC) was used as control (GeneChem). GLUT4-siRNA and negative control sequence were from GenePharma Biotechnology Company (Shanghai, China). Cells were transfected with shRNAs or siRNAs when cells reached 30-50% confluence. After 8 hours of transfection, cells were returned to normal medium in the incubator. When cells reached 85% confluence, cell supernatant, protein, and RNA were collected for later experiments.
Cell proliferation and metastasis measurement
MTT assay was used to evaluate the cell proliferation ability at 0h, 24h, 48h, 72h, and 96h after transfection (3000 cells per well). Successfully transfected cells were seeded to 6-well plate at a density of 800 cells per well. After 2 weeks, cells were fixed with paraformaldehyde and were stained with crystal violet. The number of clones with more than 50 cells was recorded and calculated. Scratch and Transwell invasion assay were used to measure cell migration and invasion. Details can be found in previous publication.18
Xenograft mouse model
Nonsilencing shRNA-transfected A375 cells, TDP-43 shRNA-2-transfected A375 cells, or TDP-43 shRNA-1-transfected A375 cells were inoculated (4 × 106/0.2ml) to right axillary fossa of each nude mice (4 in each group). Cells with transfection efficiency greater than 80% in each group were used. The size of the transplanted tumors was measured every 3 d by tumor length (L) and width (W). Tumor volume (V) was calculated by the formula V=1/2(L*W2). The mice were sacrificed 35 d after inoculation and tumor growth curve was drawn. All animal work had been proved by ethics committee.
Glucose uptake assay
For glucose uptake measurement, glucose level in cell supernatant was detected following glucose detection kit instruction (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) at 0 h and 72 h after transfection. The glucose uptake was determined by the differentials of glucose level between the 2 time points.
Immunohistochemistry
Immunohistochemistry was used to measure TDP-43 expression in paraffined samples. First antibody was anti-TDP-43 (Abnova, clone 2E2-D3, Taiwan). The methods have been described by our previous publication.19 The steps were briefly as follows: The slides were dewaxed in turpentine oil and graded alcohols, hydrated, and washed in phosphate-buffered saline. Three percent H2O2 was used to inhibit the endogenous peroxidase for 10 minutes. The slides were immersed in sodium citrate buffer and then pretreated in a microwave oven for 3 minutes. Then the slides were incubated with 10% normal goat serum for 30 minutes. Samples were incubated at 4°C overnight with anti-TDP-43 antibody (Abnova, clone 2E2-D3, Taiwan). After three washes with PBS, the sections were incubated with a biotinylated goat anti-rabbit antibody (Proteintech) for 30 mins. After horseradish peroxidase-streptavidin (Beyotime) was added to the slides, the samples were stained for 5 mins with a 0.05% 3, 3′-diaminobenzidine substrate and then counterstained for 4 mins with hematoxylin. Slides were sealed with coverslips and analyzed. Negative controls were treated identically but with the primary antibodies omitted.
Statistical analysis
Experiments were repeated 3 times, and data shown in Mean ± SD. T-test or chi-square tests were used to compare data between groups. Kaplan-Meier analysis was used to determine the survival. Data was analyzed with SPSS 19.0. P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Science Foundation of China (No. 81572689, 81372140, 81301688, 81272192, 81171882), PhD. Programs Foundation of Ministry of Education of China (No. 20130162110050 and 20130162120093), Natural Science Foundation of Hunan Province (No. 2015JJ4053, 2016JJ6154), Project of Science and Technology of Hunan Province (No. 2014SK2018 and 2015SF2066-2), Program for New Century Excellent Talents in University (NCET-11-0527), Postdoctoral Foundation of Central South University (No. 131425), “125 Talent Project” and “New Xiangya Talent Project” of the Third Xiangya Hospital of Central South University and The New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (JY201623).
References
- 1.Olszanski AJ. Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J Manag Care Spec Pharm 2014; 20:346-56; PMID:24684639; http://dx.doi.org/ 10.18553/jmcp.2014.20.4.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou J, Xu D, Xie H, Tang J, Liu R, Li J, Wang S, Chen X, Su J, Zhou X, et al.. miR-33a functions as a tumor suppressor in melanoma by targeting HIF-1alpha. Cancer Biol Ther 2015; 16:846-55; PMID:25891797; http://dx.doi.org/ 10.1080/15384047.2015.1030545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al.. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/ 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin 2010; 60:301-16; PMID:20671054; http://dx.doi.org/ 10.1017/S0009840X09991491 [DOI] [PubMed] [Google Scholar]
- 5.Cohen TJ, Lee VM, Trojanowski JQ. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med 2011; 17:659-67; PMID:21783422; http://dx.doi.org/ 10.1016/j.molmed.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YY, Kim SB, Han HD, Sohn BH, Kim JH, Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB, et al.. Tat-activating regulatory DNA-binding protein regulates glycolysis in hepatocellular carcinoma by regulating the platelet isoform of phosphofructokinase through microRNA 520. Hepatol (Baltimore, Md) 2013; 58:182-91; PMID:23389994; http://dx.doi.org/ 10.1002/hep.26310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stallings NR, Puttaparthi K, Dowling KJ, Luther CM, Burns DK, Davis K, Elliott JL. TDP-43, an ALS linked protein, regulates fat deposition and glucose homeostasis. PloS One 2013; 8:e71793; PMID:23967244; http://dx.doi.org/ 10.1371/journal.pone.0071793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warraich ST, Yang S, Nicholson GA, Blair IP. TDP-43: a DNA and RNA binding protein with roles in neurodegenerative diseases. Int J Biochem Cell Biol 2010; 42:1606-9; PMID:20601083; http://dx.doi.org/ 10.1016/j.biocel.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 9.Postel-Vinay S, Veron AS, Tirode F, Pierron G, Reynaud S, Kovar H, Oberlin O, Lapouble E, Ballet S, Lucchesi C, et al.. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat Genet 2012; 44:323-7; PMID:22327514; http://dx.doi.org/ 10.1038/ng.1085 [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Jiao F, Song Z, Li S, Liu B, Yang H, Zhou Q, Li Z. Regulation of MALAT1 expression by TDP43 controls the migration and invasion of non-small cell lung cancer cells in vitro. Biochem Biophys Res Commun 2015; 465:293-8; PMID:26265046; http://dx.doi.org/ 10.1016/j.bbrc.2015.08.027 [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Kim TK, Brozyna AA, Janjetovic Z, Brooks DL, Schwab LP, Skobowiat C, Jozwicki W, Seagroves TN. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch Biochem Biophys 2014; 563:79-93; PMID:24997364; http://dx.doi.org/ 10.1016/j.abb.2014.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao H, Wang Z, Deng Z, Ren H, Li X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int J Clin Exp Med 2015; 8:8948-57; PMID:26309547 [PMC free article] [PubMed] [Google Scholar]
- 13.Garrido P, Osorio FG, Moran J, Cabello E, Alonso A, Freije JM, Gonzalez C. Loss of GLUT4 induces metabolic reprogramming and impairs viability of breast cancer cells. J Cell Physiol 2015; 230:191-8; PMID:24931902; http://dx.doi.org/ 10.1002/jcp.24698 [DOI] [PubMed] [Google Scholar]
- 14.Dey P, Rachagani S, Chakraborty S, Singh PK, Zhao X, Gurumurthy CB, Anderson JM, Lele S, Hollingsworth MA, Band V, et al.. Overexpression of ecdysoneless in pancreatic cancer and its role in oncogenesis by regulating glycolysis. Clin Cancer Res 2012; 18:6188-98; PMID:22977192; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra RK, Wei C, Hresko RC, Bajpai R, Heitmeier M, Matulis SM, Nooka AK, Rosen ST, Hruz PW, Schiltz GE, et al.. In Silico Modeling-based Identification of Glucose Transporter 4 (GLUT4)-selective Inhibitors for Cancer Therapy. J Biol Chem 2015; 290:14441-53; PMID:25847249; http://dx.doi.org/ 10.1074/jbc.M114.628826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene 2005; 354:132-9; PMID:15978749; http://dx.doi.org/ 10.1016/j.gene.2005.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukavsky PJ, Daujotyte D, Tollervey JR, Ule J, Stuani C, Buratti E, Baralle FE, Damberger FF, Allain FH. Molecular basis of UG-rich RNA recognition by the human splicing factor TDP-43. Nat Struct Mol Biol 2013; 20:1443-9; PMID:24240615; http://dx.doi.org/ 10.1038/nsmb.2698 [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Xie H, Luo C, Chen Z, Zhou X, Xia K, Chen X, Zhou M, Cao P, Cao K, et al.. Identification of FLOT2 as a novel target for microRNA-34a in melanoma. J Cancer Res Clin Oncol 2015; 141:993-1006; PMID:25403318; http://dx.doi.org/ 10.1007/s00432-014-1874-1 [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Li J, Wen T, Zeng W, Peng C, Yan S, Tan J, Yang K, Liu S, Guo A, et al.. Overexpression of HMGB1 in melanoma predicts patient survival and suppression of HMGB1 induces cell cycle arrest and senescence in association with p21 (Waf1/Cip1) up-regulation via a p53-independent, Sp1-dependent pathway. Oncotarget 2014; 5:6387-403; PMID:25051367; http://dx.doi.org/ 10.18632/oncotarget.2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.