ABSTRACT
Out-of-field tumor response, which is also called abscopal effect, bystander effect, or non-target effect, can be regarded as localized irradiation induced systemic antitumorigenic effects, indicating shrinkage of a tumor distant from the irradiated site. Although abscopal effect has been documented in several tumor types, it is a very rare phenomenon which is clinically reported in non-small-cell-lung carcinoma (NSCLC). Herein, we present a rare case of patient with NSCLC with 2 lesions in the upper lobe of left lung who, after receiving stereotactic ablative radiation therapy (SABR) to one of the tumors, had an apparent spontaneous regression of the other mass in the lung, suggestive of a radiation-induced abscopal effect.
KEYWORDS: Abscopal effect, NSCLC, SABR, radiation, chemotherapy, target therapy, immunotherapy
Abbreviations
- NSCLC
non-small-cell-lung carcinoma
- SABR
stereotactic ablative radiation therapy
- FDG-PET/CT
fluorodeoxyglucose positron emission tomography computed tomography
- FANB
fine-needle aspiration biopsy
- EGFR
epidermal growth factor receptor
- MET
mesenchymal–epithelial transition factor
- CT
computed tomography
- DC-CIK
dendritic cells and cytokine-induced killers
Introduction
The treatment option of chemotherapy-refractory NSCLC is a dilemma for clinical oncologists for the prognosis of such patients usually leaves much to be desired. There has been a trend that cancer treatment involves multi-disciplinary team, while the best treatment scheme still requires discussing. Abscopal effect is a rare phenomenon which is probably associated with enhanced immune effect triggered by high-dose radiation. The combination of radiation, target therapy and immunotherapy might be a useful strategy for some chemotherapy-ineffective lung cancer.
Case report
In late August 2011, a 64-year-old female patient presented with right-sided chest wall lump with concurrent pain. She underwent an excision of her chest wall lump and the biopsy disclosed metastatic adenosquamous carcinoma. Fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET/CT) showed metabolic flare in the lingual segment and paramediastinum of upper lobe of left lung. After the failure of first line chemotherapy with cisplatin and pemetrexed for 6 cycles, Gefitinib was then administered in November 2012 for the EGFR exon 19 deletion mutation was identified. In late November 2014, the patient received the first dendritic cells and cytokine-induced killers (DC-CIK) immunotherapy. In December 2014, PET-CT revealed marked tumor progression in the lung. Fine-needle aspiration biopsy (FNAB) of the left lung lingual tumor revealed adenocarcinoma. Epidermal growth factor receptor (EGFR) gene mutation analysis showed exon 19 deletion mutation, exon 20 T790M mutation and no mesenchymal–epithelial transition factor (MET) amplification (Fig. 1). Thereafter, the third line systemic treatment with Pemetrexed combined with Gefitinib was administered in 3 cycles from January 2015 to March 2015. In March 2015, the patient received the second DC-CIK immunotherapy. Chest computed tomography (CT) demonstrated stable disease in the lung, revealing a lingual segment tumor measuring 6.0 × 4.9 cm and a paramediastinal tumor measuring 4.3 × 3.1 cm.
Figure 1.
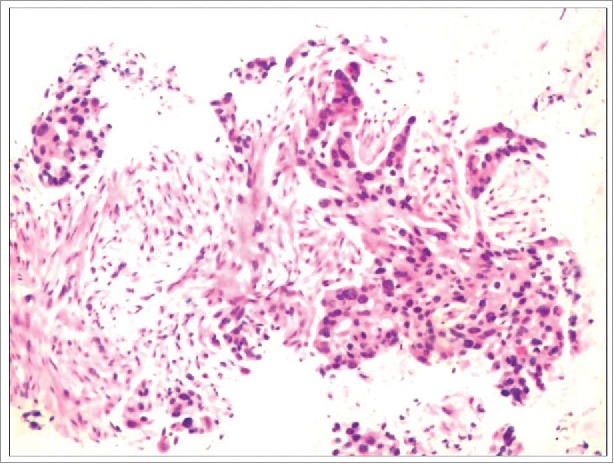
FANB of left lung mass revealed adenocarcinoma, (PAP, 200×). Immunochemistry showed CK14(−), CK7(+), P63(+), TTF-1(+).
In May 2015, a follow-up chest CT showed no changes in both lung tumors, while the patient was progressive symptomatic (cough) and could not tolerate chemotherapy. Considering the massive tumors, the patient received SABR with a dose of 37.5 Gy in 5 daily fractions for the paramediastinal foci first. Then we planned to offer SABR for the lingual foci a month later, which was declined. In September 2015, the patient was administered the third DC-CIK immunotherapy. Dramatically, in March 2016, a follow-up chest CT performed 10 months after completion of SABR showed a complete response of both pulmonary lesions. At this stage without tumor progression, AZD-9291 in place of Gefitinib was then administered as the systemic treatment. (Fig. 2)
Figure 2.
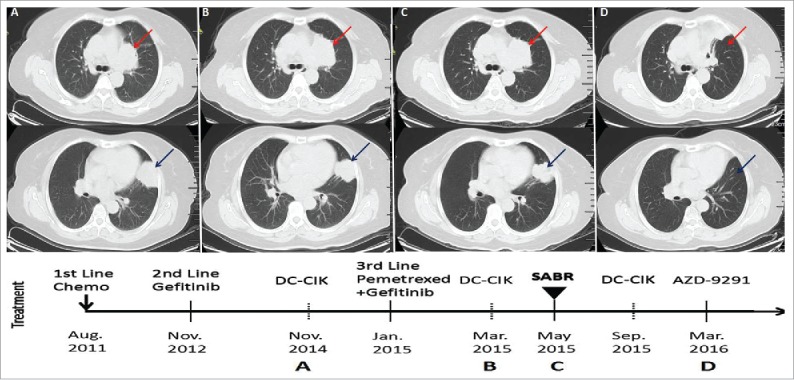
Timeline of treatment and morphologic changes in lung tumors by chest CT. Axial CT images are shown, corresponding to the timeline showing therapy and disease status. Red arrows indicate the paramediastinal mass, blue arrows indicate the lingual mass. Panel A shows images when the patient failed in 2nd line Gefitinib and was administered DC-CIK. Panel B represents the failure of 3rd line Pemetrexed plus Gefitinib and was administered DC-CIK. Panel C shows images when the patient received SABR. Panel D shows the dramatic complete response 10 months after SABR.
Discussion
Localized cancer radiotherapy may induce systemic out-of-target tumor response with a regression at unirradiated sites, suggestive of the radiation-induced abscopal effect which was first proposed over half a century ago.1 Over the past decades, the effect has been reported in several tumor types including lymphoma, melanoma, metastatic cervical carcinoma and lung metastases of hepatocellular carcinoma.2 However, clinical reports of abscopal effects in NSCLC are extremely rare.3,4 To our knowledge, ours is a rare report of the abscopal effect with focal lung radiation causing regression of distant pulmonary foci in a multiple-line systemic treatment ineffective patient with NSCLC. In this case, the lingual lobe mass, which was not the target of radiotherapy, received very low dose of radiation (667.8cGy), which supported the notion that abscopal effect was likely mediated by activation of the immune system.5,6 Postow reported a patient with melanoma had a systemic response to localized radiotherapy combined with immunotherapy with ipilimumab. Golden reported the first abscopal response which is observed after the initial treatment with ipilimumab7 and fractionated radiotherapy in a patient with chemotherapy-refractory metastatic adenocarcinoma of the lung.4 Our patient who progressed on DC-CIK had a great tumor regression after SABR, which is similar to what has been mentioned above. Therefore, we postulate the abscopal effect happened after radiotherapy and during immunotherapy.
The limitation of our report is that we can't rule out the possibility that DC-CIK immunotherapy alone may be responsible for this patient's response. Previous study reported that DC-CIK might induce an immune response against NSCLC.8 However, we cannot ignore the importance of SABR and DC-CIK immunotherapy. In our case, DC-CIK was administered 6 months before SABR and both the tumors didn't shrink, which is more supportive of an abscopal effect. More recently, research combining immune checkpoint inhibition with radiation as a systemic treatment for solid tumors has attracted much interest, which has shown a promising and remarkable clinical effect in some tumors.7,9 Our case may provide a useful strategy for chemotherapy-refractory NSCLC with a combination of radiation, target therapy and immunotherapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Mole RH. Whole body irradiation; radiobiology or medicine?. Br J Radiol 1953; 26:234-241; PMID:13042090; http://dx.doi.org/ 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 2.Burnette B, Weichselbaum RR. The immunology of ablative radiation. Semin Radiat Oncol 2015; 25(1):40-5; PMID:25481265; http://dx.doi.org/ 10.1016/j.semradonc.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Siva S, Callahan J, MacManus MP, Martin O, Hicks RJ, Ball DL. Abscopal effect after conventional and stereotactic lung irradiation of non-small-cell lung cancer. J Thorac Oncol 2013; 8(8):e71-2; PMID:23857404; http://dx.doi.org/ 10.1097/JTO.0b013e318292c55a [DOI] [PubMed] [Google Scholar]
- 4.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013; 1(6):365-72; PMID:24563870; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumniczky K, Sáfrány G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett 2015; 356(1):114-25; PMID:23994343; http://dx.doi.org/ 10.1016/j.canlet.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 6.Ng J, Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann Transl Med 2016; 4(6):118; PMID:27127771; http://dx.doi.org/ 10.21037/atm.2016.01.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al Immunologic correlates of the abscopal effect in a patient with Melanoma. N Engl J Med 2012; 366(10):925-31; PMID:22397654; http://dx.doi.org/ 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Yang X, Sun Z, Li J, Zhu H, Li J, Pang Y. Dendritic cell vaccine and cytokine-induced killer cell therapy for the treatment of advanced non-small cell lung cancer. Oncol Lett 2016; 11(4):2605-10; PMID:27073525; http://dx.doi.org/ 10.3892/ol.2016.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simone CB, Burri SH, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl Lung Cancer Res 2015; 4(5):545-52; PMID:26629423; http://dx.doi.org/ 10.3978/j.issn.2218-6751.2015.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]


