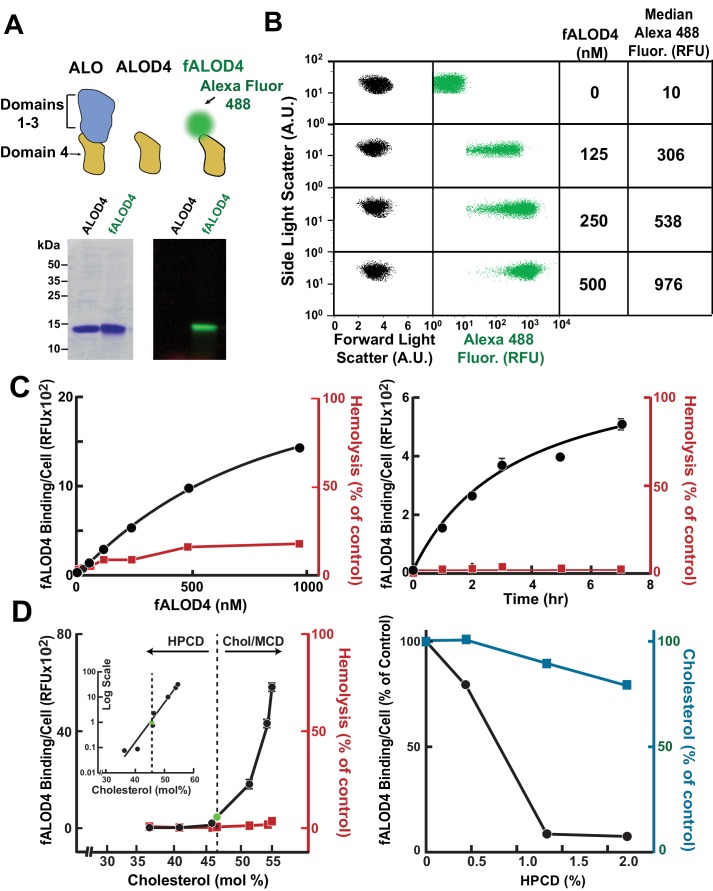
Figure 2. Assay for red blood cell (RBC) cholesterol accessibility.
(A) Schematic of anthrolysin O (ALO) domains. Domain 4 (ALOD4) binds cholesterol but does not oligomerize or form membrane-lysing pores. In fALOD4, Alexa Fluor 488 dye is covalently attached to an engineered cysteine near the NH2-terminus of domain 4. Purified proteins (5 µg) were subjected to SDS-PAGE (15%) and visualized by Coomassie staining (left) or fluorescence scanning (LI-COR) at 600 nm (right). (B) Flow cytometry analysis of fALOD4 binding to RBCs. RBCs (2.5 × 105 RBCs in 500 μl buffer D) were incubated for 3 hr at 4°C with fALOD4 at the indicated concentrations. Fluorescence was measured by a FACSCalibur flow cytometer as described in the Materials and methods. Forward light scatter (FSC), side light scatter (SSC), and Alexa 488 fluorescence measurements for 10,000 RBCs were acquired on the flow cytometer. Median Alexa 488 fluorescence per cell was calculated using FlowJo software. AU, arbitrary units; RFU, relative fluorescence units. (C) Dose response (left) and time course (right) of fALOD4 binding to RBCs. Each reaction was set up as described above using either the indicated concentrations of fALOD4 (right) or 250 nM fALOD4 (left). After incubation at 4°C for 3 hr (left) or for the indicated times (right), fALOD4 RBC binding was measured by flow cytometry. Hemolysis during fALOD4 binding reactions was determined by measuring the release of hemoglobin as described in the Materials and methods. 100% hemolysis is defined as the amount of hemoglobin released after treatment of RBCs with 1% (w/v) Triton X-100. Data points represent means of three independent measurements of the same sample (technical replicates). Error bars, which are often not visible due to the small variation, represent the SEM. The experiment was repeated three times and the results were similar. (D) Effect of RBC cholesterol modulation on RBC fALOD4 binding. (Left) RBCs were not treated (green circle) or treated with either hydroxypropyl-β-cyclodextrin (HPCD) or cholesterol/methyl-β-cyclodextrin (MCD) to reduce or increase the cholesterol content of RBCs. The fALOD4 binding assay was performed as described in the Materials and methods using 250 nM of fALOD4. Lipids were extracted from ghost membranes isolated from the RBCs, and the molar percentage of cholesterol was measured as described in the Materials and methods. The dashed line indicates the cholesterol content of untreated RBCs. Hemolysis was measured as described in the Materials and methods. Inset: Data for the experiment plotted on a logarithmic-linear scale. (Right) RBCs were treated with HPCD to remove cholesterol and both fALOD4 binding and cholesterol content were measured as described in the Materials and methods. The fALOD4 binding per cell and cholesterol content (mole %) in untreated RBCs was set to 100%. Data points represent the mean of three independent measurements of fALOD4 binding and a single measurement of RBC cholesterol content. Error bars represent the SEM. RFU, relative fluorescence units. The experiments were repeated three times and the results were similar.