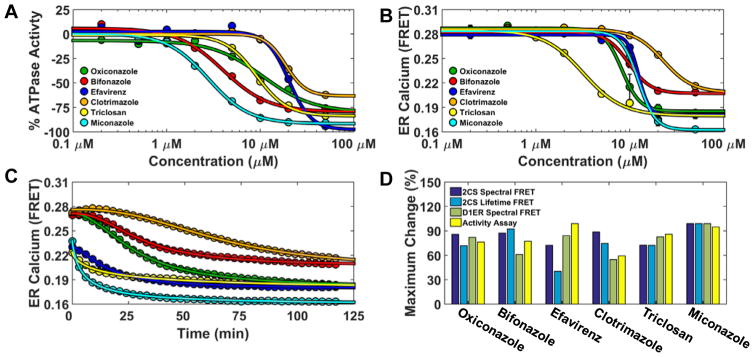
Figure 6.
Functional characterization of FRET hits on SERCA ATPase activity and ER calcium content. (A) 2CS FRET hits inhibit SERCA ATPase activity. NADH-enzyme coupled activity assay of purified SERCA was measured at eight different concentrations of the reproducible FRET hits. The maximal rate of SERCA activity was measured at saturating calcium (10 μM) after 20 minute incubation with compounds and dose-dependent inhibition was observed. (B) Endoplasmic reticulum calcium was depleted by the 2CS FRET hits. ER calcium was monitored in live-cells overexpressing the endoplasmic reticulum localized calcium FRET sensor (D1ER). D1ER FRET is dependent on calcium concentration, where less calcium causes a reduction in FRET. ER calcium levels were monitored over time in response to an eight-point concentration gradient of each hit compound. A 384 well plate was repeatedly scanned (every three minutes) with D1ER cells. The dose-dependent FRET change (ER calcium depletion) after 120 minutes compound incubation is shown and depicts differential depletion at each compound concentration. (C) Maximal ER calcium depletion in the presence of a saturating dose (50 μM) of each compound (decreased D1ER FRET) was assessed over a 120 minute period. The 2CS FRET hits displayed time-dependent and compound-specific ER calcium depletion. Miconazole (turquoise) exhibited both maximal SERCA ATPase Vmax inhibition and the largest amount of ER depletion. (D) Structure and activity assay correlation of 2CS FRET hits. The maximal change (percent change) of the structural FRET change from the 2CS FRET biosensor as well as the maximal change from two different functional assay (ATPase activity assay and D1ER calcium depletion). are shown.