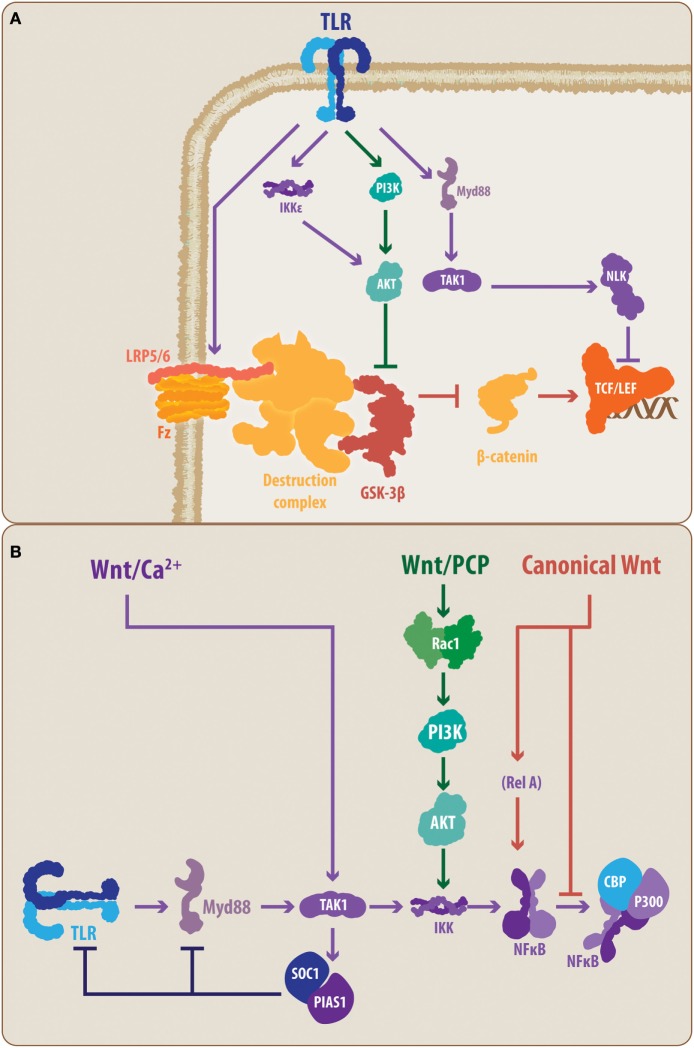
Figure 3.
Proposed molecular talking points between TLRs, Wnt, and nuclear factor-κB (NF-κB). The schematic summarizes the suggested interplaying molecular nodes between the different signaling pathways addressed in the present review. (A) According to the literature, TLRs can modulate the activity of Wnt signaling at different points. TLRs can prevent the activation of LRP6, supporting the function of the β-catenin destruction complex, thereby promoting the degradation of β-catenin and blocking the expression of Wnt target genes. In contrast, TLRs can lead, through PI3K and IKKε, to the activation of Akt, causing the inhibition of GSK3β. However, TLR signaling can also lead to NLK activation, which can interact with the Lef member of the TCF/Lef transcription factor. (B) Similarly, Wnt signaling, through its different pathways, can modulate the activity of the TLRs. The non-canonical Wnt/Ca2+ pathway can induce, through TAK1, the activation of SOC1 and PIAS1, causing a reduced expression of TLR signal transducers, such as Myd88. Similarly, the non-canonical Wnt/PCP can interact through Rac1/PI3K/Akt with the IKK complex, modulating the activation of NF-κB. In the case of canonical Wnt signaling, direct modulation of the NF-κB through a Wnt-mediated RelA interaction has been demonstrated. Moreover, it has been suggested that β-catenin can block the interaction between NF-κB and CBP/P300, preventing the transcription of NF-κB-target genes. TLR, toll-like receptor; MyD88, myeloid differentiation factor 88; TAK1, transforming growth factor-β-activated kinase-1; IKK, inhibitory NF-κB kinase; NLK, nemo-like kinase; PI3K, phosphatidylinositide-3 kinase; Akt, protein kinase B; LRP6, low-density lipoprotein receptor-related protein 6; GSK3β, glycogen synthase kinase 3 β; TCF/Lef, T-cell factor/lymphoid enhancer factor; PCP, planar cell polarity; Rac1, Ras-related C3 botulinum toxin substrate 1; SOC1, suppressor of cytokine signaling 1; PIAS1, protein inhibitors of activated STAT 1; CBP, CREB-binding protein, RelA, p65 subunit.