Abstract
AIM
To assess reference values in the literature for esophageal distensibility and cross-sectional area in healthy and diseased subjects measured by the functional lumen imaging probe (FLIP).
METHODS
Systematic search and review of articles in Medline and Embase pertaining to the use of FLIP in the esophagus was conducted in accordance with the PRISMA guidelines. Cross-sectional area and distensibility at the esophagogastric junction (EGJ) were abstracted for normal subjects, achalasia, and gastroesophageal reflux disease (GERD) patients, stratified by balloon length and volume of inflation.
RESULTS
Six achalasia studies (n = 154), 3 GERD (n = 52), and 5 studies including healthy controls (n = 98) were included in the systematic review. Normative data varied widely amongst studies of healthy volunteers. In contrast, studies in achalasia patients uniformly demonstrated low point estimates in distensibility ≤ 1.6 mm2/mmHg prior to treatment that increased to ≥ 3.4 mm2/mmHg following treatment at 40mL bag volume. In GERD patients, distensibility fell to the range of untreated achalasia (≤ 2.85 mm2/mmHg) following fundoplication.
CONCLUSION
FLIP may be a useful tool in assessment of treatment efficacy in achalasia. The drastic drop in EGJ distensibility after fundoplication suggests that FLIP measurements need to be interpreted in the context of esophageal body motility and highlights the importance of pre-operative screening for dysmotility. Future studies using standardized FLIP protocol and balloon size are needed.
Keywords: Impedance planimetry, Gastroesophageal reflux disease, Esophageal distensibility, Achalasia
Core tip: Functional lumen imaging probe (FLIP) uses impedance planimetry to calculate the distensibility of a hollow organ. In this systematic review, we aimed to assess FLIP reference values for gastroesophageal junction distensibility in healthy and diseased states. We found available normative data to vary widely. In achalasia, patients uniformly demonstrated low distensibility that improved after treatment, highlighting the role of FLIP in assessment of achalasia treatment efficacy. In gastroesophageal reflux disease, distensibility fell to the range of untreated achalasia following fundoplication, emphasizing the importance of pre-operative screening for esophageal body dysmotililty. Future studies using a standardized FLIP protocol and balloon size are needed.
INTRODUCTION
The role of the esophagus is to transport ingested material from the mouth into the stomach via complex neuromuscular activities during peristalsis. Esophageal manometry, considered the gold standard tool used to characterize esophageal motor activity, measures pressures in the form of radial squeeze amplitude at defined intervals within the esophageal body and at the lower esophageal sphincter. The technique lacks the ability to measure esophageal wall stiffness and luminal narrowing, which are important properties affecting bolus transit. At the level of the esophagogastric junction (EGJ), where flow is directly related to its ability to open in response to pressure, the biomechanical properties of the esophagus in terms of pressure-geometric data cannot be fully assessed using esophageal manometry.
Over 40 years ago, Harris et al[1] introduced the concept of measuring sphincter competence by evaluating resistance to distention rather than focusing on squeeze or tonic contraction. In 2002, Pandolfino et al[2] measured EGJ distensibility in GERD patients with hiatal hernia using a combined barostatic/fluoroscopy technique that allowed for measurement of intraluminal diameter at predetermined intraluminal distension pressures. They found that distensibility of the EGJ was significantly increased in GERD patients with hiatal hernia, likely contributing to GERD pathophysiology. In 2004, McMahon et al[3] were among the first to use impedance planimetry for measurement of EGJ competence. The investigators used a catheter with multiple electrodes and a bag mounted at the distal end that was placed at the level of the EGJ to generate cross-sectional area (CSA) and pressure data. The data was used to calculate wall tension and EGJ distensibility. Their work demonstrated the feasibility of impedance planimetry in assessing EGJ distensibility. The concept of functional luminal imaging probe (FLIP), involving identifying the narrowest region during distension plotted against bag pressure to provide a graph of compliance, was created.
In the recent years, a new imaging device, EndoFLIP (endolumenal functional lumen imaging probe, Crospon Ltd, Galway, Ireland), has become commercially available. The device consists of a long catheter with several electrodes and a bag mounted distally. Excitation current is generated between adjacent electrodes through a standardized concentration of saline injected into the bag; using impedance planimetry, CSA data are determined at the level of each pair of electrodes along the catheter. In addition, two sensors in the bag measure the intrabag pressure. The CSA-pressure data allows for calculation of distensibility (mm2/mmHg). This device has facilitated assessment of esophageal wall and EGJ distensibility, including studies in healthy volunteers and in patients with GERD, eosinophilic esophagitis, and achalasia. FLIP has also been used intraoperatively for measurements of EGJ distensibility before and after fundoplication for GERD or myotomy for achalasia. However, a major limiting factor for widespread clinical use of FLIP is the lack of a standardized protocol and reference values. A systematic review on this topic has never been performed previously. The aim of our study was to systematically assess reference values in the literature for esophageal distensibility and CSA in healthy and diseased subjects as measured by FLIP.
MATERIALS AND METHODS
We performed a systematic literature search in PubMed (National Library of Medicine) and EMBASE, including all studies published through December of 2014 that used EndoFLIP impedance planimetry to measure esophageal body or EGJ distensibility or compliance. The search terms used were (Esophagus or GERD or gastroesophageal reflux or achalasia or eosinophilic esophagitis or myotomy or dilation or hiatal hernia or fundoplication) and (FLIP or endoflip or impedance planimetry), and also using the alternative spelling “oesophagus”.
Study selection and data extraction
The studies met inclusion criteria if the following were satisfied: (1) EndoFLIP device was used; (2) EGJ or esophageal body was included in the measurement; and (3) values for distensibility/compliance, diameter, or cross-sectional area were provided. Studies involving healthy controls, GERD (pre- and post-antireflux procedures), hiatal hernia, eosinophilic esophagitis, and achalasia (pre-and post-myotomy, pneumatic dilation, or Botox) patients were included in our review. Excluded studies were those written in a language other than English, FLIP studies performed in the pediatric population or animal studies, or those done on the anorectum, small intestine, or upper esophageal sphincter. Scientific abstracts were excluded due to the lack of detailed EndoFLIP equipment and protocol information.
Study references and citations were collected in EndNote software application (Thomson Reuters, NY, United States) with duplicate publications deleted. Two investigators (Chen JW and Rubenstein JH) reviewed all titles and abstracts independently to assess eligibility. A data collection form was generated in Microsoft Excel (Microsoft, Redmond, WA, United States). For abstracts that appeared eligible on first review, both investigators independently abstracted data from the full articles. After all the data were abstracted, both investigators then compared and confirmed by consensus to account for entry error. Any discrepancies between the reviewers were resolved by joint re-review of the studies.
The database included the primary and secondary aims of the studies, study design, subject types and count, subject age/sex, parameters used to describe distension and diameter, the bag volume at which measurements were taken, location where measurements were taken, length of the FLIP balloon, and any interventions done between measurements taken. Quantitative FLIP data in terms of distension and luminal area/diameter data were recorded in the database.
Study quality criteria
The quality of each included study was assessed based on the Newcastle-Ottawa Scale. This scale measures the quality of studies on a scale of 0-9 via the assessment of three main domains: selection of study groups, comparability of groups, and comparability of groups and ascertainment of exposure. Study quality was assessed independently by both investigators and discrepancies were resolved by consensus. Studies with NOS score of 6 or above indicate good quality studies.
Statistical analysis
Included studies were separated into subject type - healthy, GERD, achalasia, and eosinophilic esophagitis (EoE) subjects. Due to the variability of study protocol in terms of balloon length and volume distension at the time of distensibility measurement, which can affect measurement values, we focused our report on the most commonly used balloon sizes and distension volumes in our analysis: EndoFLIP balloon sizes between 7 cm and 10 cm, and volume distension of 30 mL and 40 mL. Given the insufficient number of studies reporting data as mean ± SD, meta-analysis was not possible.
RESULTS
Our initial literature search identified a combined 247 citations in PubMed and Embase (Figure 1). After elimination of duplicate citations and abstract-only articles, 77 articles remained. The full-text articles of these 77 citations were reviewed and 19 studies met eligibility requirements for inclusion (Table 1). Of the 19 included studies, 8 studies were in patients with achalasia, 5 in GERD patients, 3 in eosinophilic esophagitis patients, and 11 included FLIP measurements in healthy controls. After restricting the data to balloon length (7-10 cm) and 30-40 mL volume distension, remaining were 5 studies including healthy volunteers (n = 98)[4-8], 6 including achalasia patients (n = 154)[8-13], and 3 included GERD patients (n = 52)[6,14,15]. Due to the nature of EndoFLIP as a diagnostic tool currently, studies included were non-randomized observational/cross-sectional studies. The Newcastle-Ottawa Scale (NOS) score for each study is included in Table 1. All included studies had NOS sore between 6 and 8. Four studies including healthy volunteers, two including achalasia patients, and two including GERD patients were excluded due to use of uncommon balloon length and distension volume[16-20]. FLIP studies including EoE subjects were also excluded as these 3 studies measured FLIP parameters in the esophageal body; in addition, it was unclear if FLIP measurements were taken in the distal, proximal esophagus, or both.
Figure 1.
Flow chart of records identified through literature search and those excluded and included for analysis. Initial literature search identified 247 citations. After elimination of duplicate citations and abstract-only articles, 77 articles remained. The full-text articles of these 77 citations were reviewed and 19 studies met eligibility requirements for inclusion.
Table 1.
Summary of all eligible studies
| Ref. | Quality Score | n (pre-intervention) | n (post-intervention) | Bag length (cm) | Bag volume(s) (mL) |
| Healthy | |||||
| Fukazawa et al[4], 2014 | 7 | 9 | N/A | 8 | 20, 40, 50 |
| Lin et al[18], 2013 | 6 | 10 | N/A | 16 | 30, 40, 50, 60 |
| Rieder et al[8], 2013 | 7 | 4 | N/A | 8 | 30, 40 |
| Rohof et al[19], 2012 | 7 | 15 | N/A | 14 | 50 |
| Nathanson et al[7], 2012 | 7 | 50 | N/A | 8 | 30 |
| Kwiatek et al[5], 2011 | 8 | 15 | N/A | 10 | 20, 30, 40 |
| Kwiatek et al[6], 2010 | 6 | 20 | N/A | 7 | 10, 20, 30, 40 |
| Kwiatek et al[17], 2010 | 6 | 10 | N/A | 12 | 30, 40, 50, 60 |
| Beaumont et al[16], 2009 | 5 | 8 | N/A | 10 | 60 |
| Achalasia | |||||
| Teitelbaum et al[13], 2014 | 8 | 56 | LHM (20), POEM (36) | 8 | 40 |
| Familiari et al[9], 2014 | 7 | 23 | POEM (21) | 8 | 30 |
| Teitelbaum et al[12], 2014 | 8 | 31 | LHM (12), POEM (19) | 8 | 30, 40 |
| Teitelbaum et al[11], 2013 | 8 | 25 | LHM (11), POEM (14) | 8 | 30, 40, 50 |
| Verlaan et al[20], 2013 | 8 | 10 | POEM (8) | 14 | 20, 30, 40, 50 |
| Pandolfino et al[10], 2013 | 8 | 23 | treated (31) | 10 | 20, 30, 40 |
| Rieder et al[8], 2013 | 7 | 4 | POEM (4) | 8 | 30, 40 |
| Rohof et al[19], 2012 | 7 | 30 | Treated (30) | 14 | 50 |
| GERD | |||||
| Rinsma et al[15], 2014 | 7 | 15 | TIF (15) | 8 | 30 |
| Ilczyszyn et al[14], 2014 | 8 | 17 | Nissen (17) | 8 | 30, 40 |
| Kwiatek et al[6], 2010 | 6 | 20 | N/A | 7 | 10, 20, 30, 40 |
| Kwiatek et al[17], 2010 | 6 | 10 | Fundoplicstoin (10) | 12 | 30, 40, 50, 60 |
| Beaumont et al[16], 2009 | 5 | 7 | RFA (7) | 10 | 60 |
Healthy subjects
Table 2 summarizes results from studies that reported FLIP values in healthy subjects. A total of 98 healthy subjects were included in 5 studies originating from Japan, Australia, and the United States. Healthy volunteers were recruited in each individual study for the purpose of (1) assessing the effects of mosapride on EGJ compliance[4]; (2) comparing EGJ distensibility with achalasia patients undergoing Per-oral Endoscopic Myotomy (POEM)[8]; (3) comparing EGJ distensibility with EoE patients[5]; (4) exploring EGJ distensibility during general anesthesia[7]; and (5) comparing EGJ distensibility with GERD patients[6]. Mean and median values for distensibility and CSA according to endoFLIP bag volume varied amongst studies, and these were included in Table 2. Median distensibility ranged from 0.8 mm2/mmHg to 5.7 mm2/mmHg at 30 and 40 mL balloon volumes. The wide variability in distensibility in healthy subjects is demonstrated in Figure 2A. At 40 mL volume using a 7-10 cm length balloon, the lower limit of distensibility in healthy subjects was 2.4 mm2/mmHg.
Table 2.
Healthy subjects
| Healthy | Distensibility1 (mm2/mmHg) | CSA1 (mm2) |
| 20 mL bag volume | ||
| Kwiatek et al[17], 2010 | Median 2 (5%-95% 1-9) | Median 38 (5%-95% 13-94) |
| Fukazawa et al[4], 2014 | 2.9 ± 0.6 | 25.2 ± 2.5 |
| Kwiatek et al[5], 2011 | Median 0.9 (5%-95% 0.3-1.4) | Median 15 (5%-95% 9-23) |
| 30 mL bag volume | ||
| Kwiatek et al[17], 2010 | Median 4 (5%-95% 1-14) | Median 94 (5%-95% 27-225) |
| Reider et al[8], 2013 | Median 2.5 (range 2.0-6.3) | Median 64 (range 44-91) |
| Nathanson et al[7], 2012 | Median 1.4 | Median 31 |
| Kwiatek et al[5], 2011 | Median 0.8 (5%-95% 0.4-2.8) | Median 22 (5%-95% 9-62) |
| Kwiatek et al[17], 2010 | N/A | Median 502 (5%-95% 50-68) |
| Lin et al[18], 2013 | Median 3.2 (5%-95% 1-11.6) | N/A |
| 40 mL bag volume | ||
| Kwiatek et al[17], 2010 | N/A | Median 264 (5-95% 99-496) |
| Fukazawa et al[4], 2014 | 7.1 ± 0.9 | 163 ± 5.9 |
| Rieder et al[8], 2013 | Median 2.7 (range 2.4-8.3) | Median 122 (range 73-171) |
| Kwiatek et al[6], 2010 | N/A | Median 502 (5%-95% 50-50) |
| Lin et al[18], 2013 | Median 5.7 (5%-95% 1.4-15.8) | N/A |
| 50 mL bag volume | ||
| Fukazawa et al[4], 2014 | 8.2 ± 0.8 | 259.6 ± 12 |
| Kwiatek et al[17], 2010 | N/A | Median 502 (5%-95% 50-52) |
| Rohof et al[19], 2012 | 6.3 ± 0.7 | N/A |
| Lin et al[18], 2013 | Median 5.9 (5-95% 1.6-9.3) | N/A |
| 60 mL bag volume | ||
| Beaumont et al[16], 2009 | 3.7 ± 0.93 | N/A |
| Kwiatek et al[17], 2010 | N/A | Median 93 (5%-95% 50-182) |
| Lin et al[18], 2013 | Median 6.3 (5%-95% 2.1-9.5) | N/A |
Values given in mean unless specified otherwise;
The minimal detectable CSA was 50 mm2;
Values indicate compliance (cm2/cmH2O).
Figure 2.
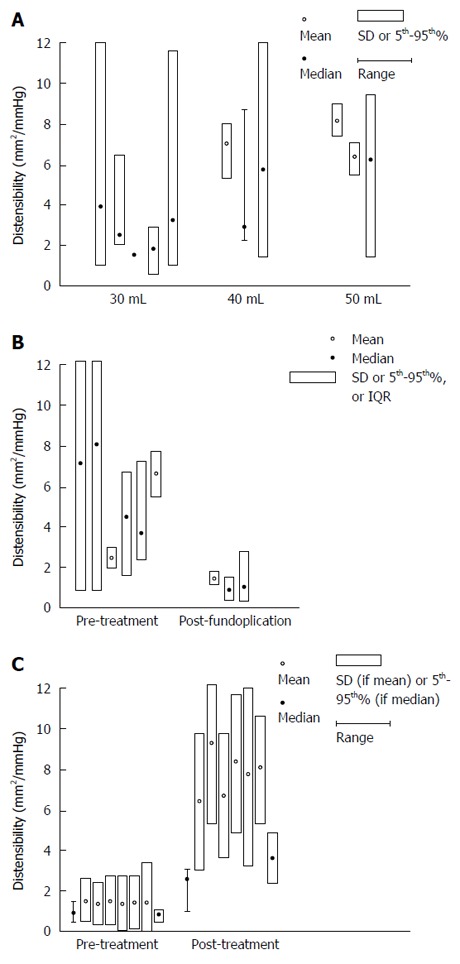
Healthy subjects. A: Mean values (open circles) with standard deviations (vertical boxes around means) and median values (closed circles) with 5th to 95th percentile (vertical boxes around medians), and ranges (vertical lines) for distensibility (mm2/mmHg) at FLIP bag volume of 30, 40, and 50 mL in healthy volunteers are shown in this plot; B: Mean values (open circles) with standard deviations (vertical boxes around means) and median values (closed circles) with 5th to 95th percentile (vertical boxes around medians), and ranges (vertical lines) for distensibility (mm2/mmHg) in GERD subjects before and after fundoplication are shown in this plot; C: Mean values (open circles) with standard deviations (vertical boxes around means) and median values (closed circles) with 5th to 95th percentile (vertical boxes around medians), and ranges (vertical lines) for distensibility (mm2/mmHg) in achalasia patients pre- and post-treatment using balloon length 7-10 cm at 40 mL bag volume are shown in this plot. The increase in EGJ distensibility post-achalasia treatment is illustrated here.
GERD subjects
Five studies included FLIP measurements in GERD patients; three of these included FLIP values measured at 30 mL volume using balloon size 7-10 cm (n = 52). Studies originated from the Netherlands, the United Kingdom, and the United States. Two of the three studies defined GERD patients using clinical symptoms as well as objective studies (endoscopy and/or pH studies)[14,15] and one study defined GERD based on clinical symptoms alone[6]. One study assessed EGJ distensibility before and after Transoral Incisionless Fundoplication (TIF) and one study assessed EGJ properties during Nissen fundoplication. Mean and Median values, standard deviation, and ranges of distensibility and CSA in GERD subjects are summarized in Table 3. Baseline/pretreatment distensibility and CSA did not appear to vary greatly compared to healthy volunteers; however, following either TIF or Nissen fundoplication, there is a reduction in distensibility. Prior to treatment, the point estimate for distensibility ranged from 2.4 to 8 mm2/mmHg at 30-40 mL bag volume. This dropped to 0.97-1.6 mm2/mmHg after fundoplication (Figure 2B).
Table 3.
Gastroesophageal reflux disease subjects
| GERD | Intervention (n) | Intra-Op (Y/N) | Pre-treatment Distensibility1 (mm2/mmHg) | Post-treatment Distensibility1 (mm2/mmHg) | Pre-Treatment CSA1 (mm2) | Post-treatment CSA1 (mm2) |
| 20 mL bag volume | ||||||
| Kwiatek et al[5], 2010 | N/A | N | Median 7 (5%-95% 1-32) | N/A | Median 36 (5%-95% 13-201) | N/A |
| 30 mL bag volume | ||||||
| Kwiatek et al[6], 2010 | N/A | N | Median 8 (5%-95% 1-46) | N/A | Median 116 (5%-95% 33-344) | N/A |
| Rinsma et al[15], 2014 | TIF (15) | Y | 2.4 ± 0.3 | 1.6 ± 0.2 | 40.6 ± 5.4 | 33.6 ± 4.1 |
| Ilczyszyn et al[14], 2014 | Nissen (17) | Y | Median 4.23 (IQR 1.60-6.58) | Median 0.972 (IQR 0.715-1.72) | Median 75.1 | |
| Kwiatek et al[17], 2010 | Fundoplication (10) | N | N/A | N/A | N/A | Median 51 (5%-95% 50-60) |
| 40 mL bag volume | ||||||
| Kwiatek et al[17], 2010 | N/A | N | N/A | N/A | Median 180 (5%-95% 86-409) | N/A |
| Ilczyszyn et al[14], 2014 | Nissen (17) | Y | Median 3.75 (IQR 2.43-7.21) | Median 1.36 (IQR 0.537-2.85) | Median 124.2 | N/A |
| Kwiatek et al[17], 2010 | Fundoplication (10) | N | N/A | N/A | N/A | Median 51 (5%-95% 50-56) |
| 50 mL bag volume | ||||||
| Kwiatek et al[17], 2010 | Fundoplication (10) | N | N/A | N/A | N/A | Median 61 (5%-95% 52-88) |
| 60 mL bag volume | ||||||
| Beaumont et al[16], 2009 | Radiofrequency ablation | N | 6.5 ± 0.92 | 7.3 ± 1.32 | N/A | N/A |
| Kwiatek et al[17], 2010 | Fundoplication (10) | N | N/A | N/A | N/A | Median 159 (5%-95% 68-245) |
Values given in mean unless specified otherwise;
Values indicate compliance (cm2/cmH2O). GERD: Gastroesophageal reflux disease.
Achalasia subjects
EndoFLIP balloon size 7-10 cm at 30-40 mL was used in 6 of 8 studies (n = 154) to assess treatment response in achalasia patients[8-13,19,20]. Treatment for achalasia included laparoscopic Heller myotomy (LHM), POEM, and unspecified (including LHM, POEM, and pneumatic dilation). Distensibility and CSA before and after treatment are listed in Table 4. At 30 mL volume distension, the point estimates for distensibility in achalasia pre-treatment (0.8-2.2 mm2/mmHg) overlapped with that in healthy volunteers (0.8-4.0 mm2/mmHg), as did the point estimates for CSA (22 and 32.9 mm2 for achalasia vs 22-94 for healthy volunteers). However, at 40 mL volume, there was a clear difference in distensibility between achalasia (point estimates ≤ 1.6 mm2/mmHg) and healthy volunteers (point estimates 2.7 to 7.1 in 3 studies) and for CSA (≤ 41.5 mm2 for achalasia vs ≥ 122.3 mm2 for healthy volunteers). Comparing to pre-treatment, all but one small study (n = 4) demonstrated post-treatment distensibility of 2.2 mm2/mmHg or higher (point estimate of ≥ 3.4 mm2/mmHg), and CSA of 86 mm2 or higher regardless of the treatment modality. Figure 2C illustrates the rise in EGJ distensibility post-achalasia treatment at 40mL bag volume using balloon length 7-10 cm.
Table 4.
Achalasia subjects
| Achalasia | Intervention (n) | Pre-treatment Distensibility1 (mm2/mmHg) | Post-treatment Distensibility1 (mm2/mmHg) | Pre-treatment CSA1 (mm2) | Post-treatment CSA1 (mm2) |
| 20 mL bag volume | |||||
| Pandolfino et al[10], 2013 | Untreated (23) | Median 1.1 (5%-95% 0.9-1.6) | Median 1.8 (5%-95% 1.2-2.2) | N/A | N/A |
| Treated2 (17) | |||||
| Verlaan et al[20], 2013 | 10 | Median 1.4 (IQR 1.1-2.4) | POEM: Median 3.0 (IQR 1.4-9.4) | N/A | N/A |
| 30 mL bag volume | |||||
| Familiari et al[9], 2014 | POEM (21) | N/A | N/A | 32.9 ± 23.1 | 102.38 ± 28.2 |
| Teitelbaum et al[11], 2014 | LHM (12) | LHM: 2.2 ± 1.7 | LHM: 6.9 ± 3.3 | N/A | N/A |
| POEM (19) | POEM: 1.8 ± 1.4 | POEM: 9.3 ± 4.1 | |||
| Teitelbaum et al[12], 20133 | LHM (11) | LHM: 1.7 ± 1.5 | LHM: 6.7 ± 4.4 | N/A | N/A |
| POEM (14) | POEM: 1.8 ± 1.1 | POEM: 8.2 ± 3.0 | |||
| Rieder et al[8], 2013 | POEM (4) | Median 0.8 (range 0.7-1.0) | Median 3.1 (range 1.7-3.4) | Median 22 (range 20-32) | Median 71.5 (range 30-106) |
| Pandolfino et al[10], 2013 | Untreated (23) | Median 1.0 (5%-95% 0.8-1.2) | Median 2.5 (5%-95% 1.3-3.4) | N/A | N/A |
| Treated2 (17) | |||||
| Verlaan et al[20], 2013 | 10 | Median 1.0 (IQR 0.8-1.5) | POEM: Median 2.9 (IQR 1.3-19.6) | N/A | N/A |
| 40 mL bag volume | |||||
| Rieder et al[8], 2013 | POEM (4) | Median 1.0 (range 0.5-1.4) | Median 2.4 (range 1.1-3.0) | Median 41.5 (range 20-49) | Median 86 (range 41-137) |
| Teitelbaum et al[11], 2014 | LHM (12) | LHM: 1.6 ± 1.0 | LHM: 6.3 ± 3.4 | N/A | N/A |
| POEM (19) | POEM: 1.3 ± 1.0 | POEM: 9.2 ± 3.9 | |||
| Teitelbaum et al[12], 2014 | LHM (20) | LHM: 1.5 ± 1.2 | LHM 6.6 ± 3.1 | N/A | N/A |
| POEM (36) | POEM: 1.3 ± 1.4 | POEM 8.3 ± 3.4 | |||
| Teitelbaum et al[13], 20133 | LHM (11) | LHM: 1.4 ± 1.3 | LHM: 7.6 ± 4.4 | LHM 33.5 | LHM 163.6 |
| POEM (14) | POEM: 1.4 ± 1.9 | POEM: 7.9 ± 2.7 | POEM 38.8 | POEM 163.3 | |
| Pandolfino et al[10], 2013 | Untreated (23) | Median 0.7 (5%-95% 0.5-1.1) | Median 3.4 (5%-95% 2.2-4.9) | N/A | N/A |
| Treated (17) | |||||
| Verlaan et al[20], 2013 | 10 | Median 1.1 (IQR 0.55-2.0) | POEM: Median 4.0 (IQR 2.9-12.7) | N/A | N/A |
| 50 mL bag volume | |||||
| Teitelbaum et al[11], 20133 | LHM (11) | LHM: 1.1 ± 0.9 | LHM: 5.7 ± 3.1 | N/A | N/A |
| POEM (14) | POEM: 1.4 ± 2.1 | POEM: 6 ± 1.5 | |||
| Rohof et al[19], 2012 | 7 Treated (6 PD, 1 LHM) | 0.7 ± 0.9 | 4.4 ± 0.5 | N/A | N/A |
| Verlaan et al[20], 2013 | 10 | Median 1.0 (IQR 0.4-2.3) | POEM: Median 6.7 (IQR 3.8-16.6) | N/A | N/A |
Values given in mean unless specified otherwise;
Treated included patients that underwent pneumatic dilation, Heller myotomy with partial fundoplication, and POEM with good response;
Subjects were included in the more recent Teitelbaum study (2014). CSA: Cross-sectional area; LHM: Laparoscopic Heller Myotomy; POEM: Peroral endoscopic myotomy; PD: Pneumatic dilation.
DISCUSSION
It has become increasing clear that a clinical tool measuring distensibility (essentially resistance to distension), rather than pressure, is useful in assessing the function of EGJ[21]. FLIP offers information on distensibility in the esophagus and can potentially provide information on sphincter dynamics during distension to help distinguish between normal sphincters, patients with reflux disease, and patients with achalasia[22]. This tool has recently become commercially available and has been used to study the biomechanics of esophageal body and sphincter in healthy subjects and in patients with GERD, achalasia, eosinophilic esophagitis, and others. One major factor limiting its widespread use is the lack of reference values for differentiation of normal and diseased conditions. To date, there has not been a systematic review on FLIP measurements in healthy and diseased states. Our study aimed to summarize the currently available FLIP data on healthy subjects and the patient population.
We found that normative data varied widely amongst studies of healthy volunteers. This is potentially due to the variability in balloon sizes and FLIP protocols used, as well as the variable definition of normal subjects. Additionally, Tucker et al[23] have demonstrated an inverse association between FLIP measurements (CSA and distensibility) and Body Mass Index (BMI). BMI information was not available and therefore was not controlled in the studies included in this review and may have contributed to the variability of the normative data.
The available data suggests that use of an 8cm balloon (marketed as the 325N catheter) at 40 mL volume is the ideal protocol for assessing EGJ distensibility. In that protocol, healthy patients had distensibility greater than 2.4 mm2/mmHg, and patients with untreated achalasia typically had distensibility less than 3.0 mm2/mmHg with a median of 1.3 mm2/mmHg. Following treatment for achalasia, those patients typically had distensibility greater than 3.0 mm2/mmHg with a median of 7.6 mm2/mmHg. This suggests that FLIP may be a useful tool in assessment of treatment adequacy intra-operatively or on follow-up of achalasia patients. The role of intra-operative FLIP measurement was highlighted in a study that showed that an extended proximal myotomy was required to normalize distensibility during LHM[12].
CSA and distensibility in GERD patients prior to treatment did not appear to differ from healthy volunteers; however, following anti-reflux procedures, distensibility fell to the range of untreated achalasia (≤ 1.6 mm2/mmHg). The drastic drop in EGJ distensibility in GERD patients after antireflux procedures suggests that FLIP EGJ measures should not be interpreted in isolation from data on esophageal body motility, as most of these post-antireflux surgery patients do not exhibit impaired esophageal emptying to the degree of achalasia. This finding also highlights the importance of screening for esophageal motility disorders prior to fundoplication to avoid the heightened risk of pseudoachalasia.
Our systematic review was limited by the overall small number of studies using FLIP to assess distensibility of the esophagus in a heterogeneous population of subjects. There is also a wide variability in the size/length of balloon used and FLIP protocol from study to study. Data comparison was also made difficult due to the variable FLIP parameters reported (e.g., CSA, distensibility, distensibility index, distensibility plateau, etc.). Interpretation of distensibility was also made difficult due to the likelihood of non-normally distributed data, as evident by standard deviation larger than mean in several of the studies. Since some studies reporting data as mean ± SD and others reporting median (range), meta-analysis could not be performed.
FLIP provides valuable information regarding esophageal wall compliance and lower esophageal sphincter competency that complement other diagnostic tools such as esophageal manometry and barium esophagram. From our systematic review of the literature, FLIP may especially have a role in assessment of treatment response in patients with achalasia or GERD patients undergoing intervention. However, future studies in larger number normal subjects and patients using standardized FLIP protocol and balloon size are needed for reliable interpretation of FLIP data. In the meantime, use of an 8 cm balloon at 40 mL volume is the most likely clinically relevant protocol for distinguishing achalasia from normal esophagus, and assessing response to therapy.
COMMENTS
Background
The functional lumen imaging probe (FLIP) has been used to assess esophagogastric junction (EGJ) distensibility; however, its routine use in clinical practice is limited by the lack of established reference values.
Research frontiers
FLIP has been used in research studies to assess the distensibility of esophageal wall and gastroesophageal junction in healthy volunteers and in patients with GERD, eosinophilic esophagitis, and achalasia. However, the clinical role of FLIP is currently still being investigated. Prospective outcome studies using FLIP technology are needed.
Innovations and breakthroughs
A systematic review of the currently available FLIP data in the literature had not been done prior to this study.
Applications
This study demonstrated the potential utility of FLIP in assessment of achalasia treatment efficacy. It also highlighted the importance of future studies using a standardized FLIP protocol and balloon size.
Terminology
Impedance planimetry: an imaging technique involving a conductive fluid-filled bag on a catheter with multiple impedance and pressure sensors. When the catheter-bag device is placed in a hollow tube, impedance measurements between pairs of electrodes are used to estimate the cross-sectional area of the tube. With simultaneous measurement of intrabag pressure, distensibility (smallest cross sectional area/bag pressure) is calculated. EndoFLIP (endolumenal functional lumen imaging probe): A new technology that uses impedance planimetry to measure the cross sectional area and pressure in a hollow organ to determine its distensibility.
Peer-review
Chen and Rubenstein wrote a systematic review of literature concerning the use of FLIP system to evaluate EGJ in achalasia, GERD patients undergoing surgical procedure and healthy controls. The review is well written and it addresses a novel field with possible evolution in the future. The research strategy is adequate and adherent to the standard of quality.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Chen JW and Rubenstein JH have no commercial, personal, political, intellectual, or religious conflict of interest.
Data sharing statement: Technical appendix and dataset available from the corresponding author at chenjoan@med.umich.edu.
Peer-review started: October 9, 2016
First decision: November 9, 2016
Article in press: December 21, 2016
P- Reviewer: Ierardi E, Nicodeme F, Tolone S, Wang BM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Harris LD, Pope CE. “Squeeze” vs. resistance: an evaluation of the mechanism of sphincter competence. J Clin Invest. 1964;43:2272–2278. doi: 10.1172/JCI105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Shi G, Curry J, Joehl RJ, Brasseur JG, Kahrilas PJ. Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1052–G1058. doi: 10.1152/ajpgi.00279.2001. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BP, Frøkjaer JB, Drewes AM, Gregersen H. A new measurement of oesophago-gastric junction competence. Neurogastroenterol Motil. 2004;16:543–546. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 4.Fukazawa K, Furuta K, Adachi K, Moritou Y, Saito T, Kusunoki R, Uno G, Shimura S, Aimi M, Ohara S, et al. Effects of mosapride on esophageal motor activity and esophagogastric junction compliance in healthy volunteers. J Gastroenterol. 2014;49:1307–1313. doi: 10.1007/s00535-013-0876-0. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatek MA, Pandolfino JE, Hirano I, Kahrilas PJ. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72:272–278. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathanson LK, Brunott N, Cavallucci D. Adult esophagogastric junction distensibility during general anesthesia assessed with an endoscopic functional luminal imaging probe (EndoFLIP®) Surg Endosc. 2012;26:1051–1055. doi: 10.1007/s00464-011-1996-3. [DOI] [PubMed] [Google Scholar]
- 8.Rieder E, Swanström LL, Perretta S, Lenglinger J, Riegler M, Dunst CM. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc. 2013;27:400–405. doi: 10.1007/s00464-012-2484-0. [DOI] [PubMed] [Google Scholar]
- 9.Familiari P, Gigante G, Marchese M, Boskoski I, Bove V, Tringali A, Perri V, Onder G, Costamagna G. EndoFLIP system for the intraoperative evaluation of peroral endoscopic myotomy. United European Gastroenterol J. 2014;2:77–83. doi: 10.1177/2050640614521193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfino JE, de Ruigh A, Nicodème F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25:496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teitelbaum EN, Boris L, Arafat FO, Nicodème F, Lin Z, Kahrilas PJ, Pandolfino JE, Soper NJ, Hungness ES. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc. 2013;27:4547–4555. doi: 10.1007/s00464-013-3121-2. [DOI] [PubMed] [Google Scholar]
- 12.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Boris L, Nicodème F, Lin Z, Hungness ES. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc. 2014;28:2840–2847. doi: 10.1007/s00464-014-3563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Hirano I, Boris L, Nicodème F, Lin Z, Hungness ES. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522–528. doi: 10.1007/s00464-014-3733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus. 2014;27:637–644. doi: 10.1111/dote.12130. [DOI] [PubMed] [Google Scholar]
- 15.Rinsma NF, Smeets FG, Bruls DW, Kessing BF, Bouvy ND, Masclee AA, Conchillo JM. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc. 2014;28:941–949. doi: 10.1007/s00464-013-3250-7. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont H, Gondrie JJ, McMahon BP, Pouw RE, Gregersen H, Bergman JJ, Boeckxstaens GE. Stepwise radiofrequency ablation of Barrett’s esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy. 2009;41:2–8. doi: 10.1055/s-0028-1103451. [DOI] [PubMed] [Google Scholar]
- 17.Kwiatek MA, Kahrilas K, Soper NJ, Bulsiewicz WJ, McMahon BP, Gregersen H, Pandolfino JE. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14:268–276. doi: 10.1007/s11605-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Nicodème F, Boris L, Lin CY, Kahrilas PJ, Pandolfino JE. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterol Motil. 2013;25:e765–e771. doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 20.Verlaan T, Rohof WO, Bredenoord AJ, Eberl S, Rösch T, Fockens P. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78:39–44. doi: 10.1016/j.gie.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Moonen A, Boeckxstaens G. Measuring mechanical properties of the esophageal wall using impedance planimetry. Gastrointest Endosc Clin N Am. 2014;24:607–618. doi: 10.1016/j.giec.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 22.McMahon BP, Rao SS, Gregersen H, Kwiatek MA, Pandolfino JE, Drewes AM, Krarup AL, Lottrup C, Frøkjaer JB. Distensibility testing of the esophagus. Ann N Y Acad Sci. 2011;1232:331–340. doi: 10.1111/j.1749-6632.2011.06069.x. [DOI] [PubMed] [Google Scholar]
- 23.Tucker E, Sweis R, Anggiansah A, Wong T, Telakis E, Knowles K, Wright J, Fox M. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25:904–910. doi: 10.1111/nmo.12218. [DOI] [PubMed] [Google Scholar]


