Abstract
R-ketamine appears to be a potent, long-lasting and safer antidepressant, relative to esketamine (S-ketamine), since it might be free of psychotomimetic side effects. Using [11C]raclopride and positron emission tomography (PET), we investigated whether esketamine and R-ketamine can affect dopamine D2/3 receptor binding in the conscious monkey brain. A single infusion of esketamine (0.5 mg/kg), but not R-ketamine (0.5 mg/kg), caused a reduction of binding availability of dopamine D2/3 receptor in the monkey striatum. This study suggests that unlike to R-ketamine, esketamine can cause dopamine release in the striatum, and that its release might be associated with psychotomimetic effects of esketamine.
Keywords: Dopamine D2/3 receptor, Esketamine, Release, R-ketamine, Monkey
Introduction
The rapid-onset antidepressant effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine have attracted serious attention after it was found that a single sub-anesthetic dose (0.5 mg/kg) of ketamine elicited a rapid antidepressant effect within 1–2 h, in depressed patients, including those with treatment-resistant depression and treatment-resistant bipolar depression [1–4]. These beneficial effects persist for up to 2 weeks in some patients. A recent meta-analysis demonstrated that ketamine produced a rapid, yet transient, antidepressant effect, with odds ratios for response and transient remission of symptoms at 24 h equaling 9.87 and 14.7, respectively, accompanied by brief psychotomimetic and dissociative effects [5].
Ketamine (or RS-ketamine) is a racemic mixture containing equal parts of R-ketamine and S-ketamine (esketamine). Esketamine shows an approximately threefold to fourfold greater anesthetic potency and greater undesirable psychotomimetic side effects, compared with R-ketamine [6]. This is related to the fact that esketamine has an approximately fourfold greater affinity for the NMDA receptor relative to R-ketamine [6]. We reported that R-ketamine shows greater potency and longer-lasting antidepressant effects than esketamine in animal depression models, including neonatal dexamethasone exposure, repeated social defeat stress and learned helplessness, and that unlike esketamine, R-ketamine does not induce psychotomimetic side effects and abuse potential in rodents [7–9]. In addition, we reported that a single dose of esketamine (10 mg/kg), but not R-ketamine (10 mg/kg), resulted in loss of parvalbumin (PV)-positive cells in mouse brain regions, such as the medial prefrontal cortex [8], suggesting that loss of PV-positive cells may be associated with psychosis.
Dopamine D2/3 receptors have a high-affinity state for endogenous dopamine and low-affinity state. Raclopride is a moderately high-affinity selective antagonist at these states of the dopamine D2/3 receptors. [11C]Raclopride has been used as a positron emission tomography (PET) ligand for characterization dopamine D2/3 receptors in the brain from human and monkey. Interestingly, PET using [11C]raclopride would be useful for detection of release of endogenous dopamine from presynaptic terminal [10]. Given differential effects of ketamine enantiomers in the brain, the present study using PET was performed to examine whether a single infusion of ketamine enantiomers could affect the release of endogenous dopamine in the conscious monkey brain.
Methods
Animals and drugs
Experiments were conducted in accordance with the recommendations of the US National Institutes of Health. The following experiments were approved by the Ethical Committee of the Central Research Laboratory, Hamamatsu Photonics (Hamamatsu, Shizuoka, Japan). Four male rhesus monkeys (Macaca mulatta; 7.1 ± 1.3 years old, weighing 7.2 ± 1.1 kg) were studied 3 times (saline, esketamine and R-ketamine). The order of PET scan was saline, esketamine and R-ketamine with 2-week period between each scan. The doses (0.5 mg/kg) of esketamine or R-ketamine hydrochloride were based on a previous monkey study [11] and human studies [1–4]. Esketamine hydrochloride and R-ketamine hydrochloride were synthesized by K.H. at Chiba University (Chiba, Japan) and were dissolved in physiological saline.
Preparation of [11C]raclopride and PET experiments
[11C]Raclopride was labeled by N-methylation of respective nor compound with [11C]methyl triflate prepared from [11C]methyl iodide. The radioactive purity of [11C]raclopride was greater than 98 %, and the specific radioactivity was 32.8 ± 5.6 GBq/μmol (mean ± SD, n = 12). A high-resolution animal PET scanner (SHR-7700; Hamamatsu Photonics, Hamamatsu, Japan) with a transaxial resolution of 2.6-mm full width half maximum in the enhanced 2D mode and a center-to-center distance of 3.6 mm [12] was used. PET images were reconstructed by a filtered back projection method with a 4.5-mm Hanning filter, resulting in an in-plane reconstructed resolution of 4.5 mm. PET scans with [11C]raclopride were performed without arterial blood sampling. The trained animal’s head was rigidly fixed to the upper frame of a monkey chair using an acrylic head-restraining device. The animal sitting in a restraining chair was placed at a fixed position in the PET gantry with stereotactic coordinates aligned parallel to the orbitomeatal line. Transmission data with a 68Ge–68Ga pin source were obtained for an attenuation correction.
[11C]Raclopride was administered intravenously after the end of ketamine infusion (0.5 mg/kg for 40 min), and PET scans were also started after the end of ketamine infusion. Vital signs including heart rate, respiration rate, systolic and diastolic blood pressure, and body temperature were monitored throughout the infusion of ketamine enantiomers. PET scans were acquired for 91 min after intravenous bolus injection of [11C]raclopride. The injected dose of [11C]raclopride was 164.2 ± 15.9 MBq/kg (mean ± SD, n = 12). A summation image from 61 to 91 min post-injection was obtained (Fig. 1). The regions of interest (ROIs) were drown on the individual MRI in bilateral caudates and putamens, and cerebellum, and automatically copied and pasted them on the corresponding PET image slices, then converted these ROIs into one VOI using PMOD. The time-activity curves obtained from the VOI were applied for simplified reference tissue model (SRTM) analysis. The quantitative analysis of [11C]raclopride was performed with SRTM in order to calculate the non-displaceable binding potential (BPND) [13] using the time-activity curve in the cerebellum as an input function.
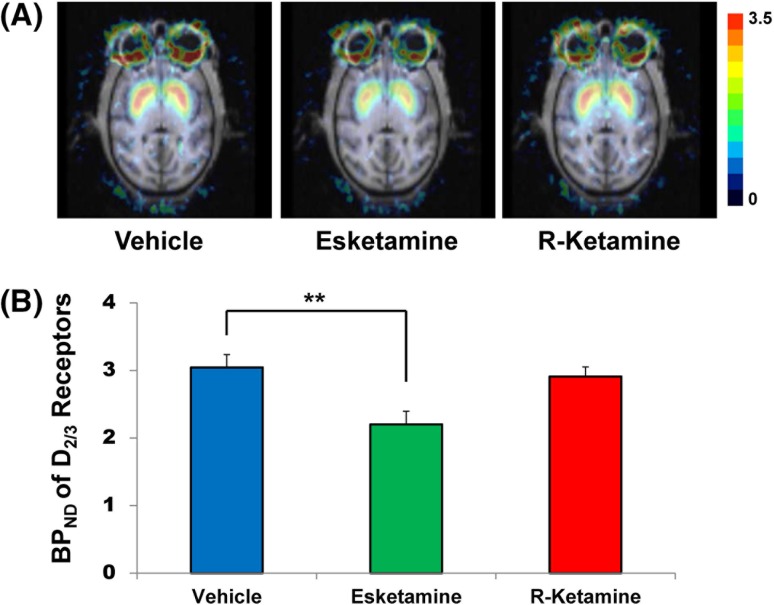
Fig. 1.
Effect of esketamine and R-ketamine on the BPND of [11C]raclopride to dopamine D2/3 receptors in the striatum of conscious monkeys. a Representative photomicrographs of typical MRI and parametric PET images of [11C]raclopride to dopamine D2/3 receptors from vehicle (saline)-treated, esketamine (0.5 mg/kg, 40-min)-treated and R-ketamine (0.5 mg/kg, 40-min)-treated monkeys. PET images from 61 to 91 min were obtained. Color bar indicates a level of the non-displaceable binding potential (BPND) of [11C]raclopride. b The non-displaceable binding potential BPND of [11C]raclopride in the striatum. The data show the mean ± S.E.M. (n = 4). **P < 0.01 compared with vehicle (saline)-treated condition
Statistical analysis
The data are shown as the mean ± standard error of the mean (S.E.M.). Analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS). Comparisons between groups were made using the repeated-measures analysis of variance (ANOVA), followed by post hoc Bonferroni test. The P values of less than 0.05 were considered statistically significant.
Results
Marked accumulation of radioactivity in the striatum was shown after an intravenous administration of [11C]raclopride (Fig. 1). Radioactivity in the striatum of esketamine-treated monkeys was lower than those of vehicle (saline)-treated monkeys (Fig. 1). Repeated-measures ANOVA demonstrated a significant change among three conditions in the striatum (F = 70.252, P < 0.001). Post hoc Bonferroni test revealed that a single infusion of esketamine significantly (P = 0.004) decreased BPND in the striatum of monkey brain (Fig. 1). In contrast, a single infusion of R-ketamine did not decrease BPND in the striatum of monkey brain (P = 0.603) (Fig. 1).
Discussion
Using a conscious PET study, we found that a single infusion of esketamine, but not R-ketamine, elicited a significant reduction of BPND of dopamine D2/3 receptor in the striatum of monkeys. Since [11C]raclopride-PET has been used for detection of release of endogenous dopamine from presynaptic terminal [10], this study suggests that esketamine, but not R-ketamine, causes the marked release of endogenous dopamine from presynaptic terminal in the striatum of monkeys.
Clinical use of ketamine is limited due to its side effects such as psychotomimetic effects [14]. Unlike esketamine, R-ketamine might not appear to cause psychotomimetic effects, based on the lack of behavioral abnormalities (e.g., hyperlocomotion, prepulse inhibition deficits) observed in rodents after treatment [8]. Recently, Singh et al. [15] reported a rapid-onset antidepressant effect of esketamine in treatment-resistant patients with depression although Brief Psychiatric Rating Scale (BPRS) score and Clinician Administered Dissociative States Scale (CADSS) score were the highest at 40 min after an infusion of esketamine (0.20 or 0.40 mg/kg for 40 min). Furthermore, an infusion of esketamine, but not R-ketamine, in healthy subjects produced a dissociative state and psychotic syndrome, including disturbances of emotion and sensory perception, difficulties in thinking and reality appraisal, as well as ego disorders [16]. Subsequently, Vollenweider et al. [17] reported that an infusion of esketamine caused the reduction of in vivo binding of [11C]raclopride binding to dopamine D2/3 receptors in human brain, indicating an increase of dopamine in the striatum. Taken together, it is likely that esketamine-associated psychotomimetic effects in patients might be associated with marked dopamine release in the striatum. Given the role of dopamine release in the striatum for ketamine (or esketamine)-induced psychotomimetic effects, it is unlikely that R-ketamine might cause psychotomimetic effects in monkeys or humans.
Despite an increasing number of studies focusing on the rapid antidepressant effects of ketamine in treatment-resistant depression, its potential to elicit abuse liability cannot be ignored [14]. In contrast, a schedule of repeated ketamine infusions could provide effective management of depressive symptoms in patients with treatment-resistant depression [15]. A study using the conditioned place preference (CPP) test showed that ketamine (1.0–10 mg/kg) significantly increased CPP scores in mice, in a dose-dependent manner [18], suggesting that ketamine has rewarding effects. We also reported that repeated administration of esketamine (5, 10 or 20 mg/kg), but not R-ketamine (5, 10 or 20 mg/kg), significantly increased CPP scores in mice, in a dose-dependent manner [8]. Taken together, it is possible that release of dopamine by esketamine could induce rewarding effects in rodents [8]. Given the role of dopamine release in the striatum for ketamine-induced rewarding effects, it is unlikely that R-ketamine might cause rewarding effects in monkeys or humans. These findings suggest greater safety for repeated intermittent dosing with R-ketamine relative to esketamine.
In conclusion, this study demonstrates that a single infusion of esketamine, but not R-ketamine, could cause a marked reduction of BPND of dopamine D2/3 receptor in the striatum of conscious monkeys. Therefore, relative to esketamine, R-ketamine is likely to be a safer antidepressant in the treatment of depression.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H., #24116006).
Compliance with ethical standards
Conflict of interest
Dr. Hashimoto is an inventor on a filed patent application on “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. K.H. has served as a scientific consultant to Astellas, Dainippon-Sumitomo and Taisho, and he has also received research support from Abbvie, Dainippon-Sumitomo, Mochida, Otsuka and Taisho. The remaining authors declare no conflicts of interest.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, APA Council of Research Task Force on Novel Biomarkers and Treatments Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–956. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 6.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JC, Li SX, Hashimoto K. R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K. The R-stereoisomer of ketamine as an alternative for ketamine for treatment-resistant major depression. Clin Psychopharmacol Neurosci. 2015;12:72–73. doi: 10.9758/cpn.2014.12.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Cholinergic neuronal modulation alters dopamine D-2 receptor availability in vivo by regulating receptor affinity induced by facilitated synaptic dopamine turnover? Positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci. 2000;20:7067–7073. doi: 10.1523/JNEUROSCI.20-18-07067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, Domino EF. Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: a PET study in conscious monkeys. Neuropsychopharmacology. 2013;38:2666–2674. doi: 10.1038/npp.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe M, Okada H, Shimizu K, Omura T, Yoshikawa E, Kosugi T, Mori S, Yamashita T. A high resolution animal PET scanner using compact PS-PET detectors. IEEE Trans Nucl Sci. 1997;44:1277–1282. doi: 10.1109/23.597001. [DOI] [Google Scholar]
- 13.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology. 2014;231:2041–2042. doi: 10.1007/s00213-014-3543-0. [DOI] [PubMed] [Google Scholar]
- 15.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/S0924-977X(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 17.Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res. 2000;34:35–43. doi: 10.1016/S0022-3956(99)00031-X. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Aoki T, Kato H, Yamazaki M, Misawa M. Effects of the 5-HT3 receptor antagonist ondansetron on the ketamine-and dizocilpine-induced place preferences ion mice. Eur J Pharmacol. 1999;385:99–102. doi: 10.1016/S0014-2999(99)00762-1. [DOI] [PubMed] [Google Scholar]