Figure 2.
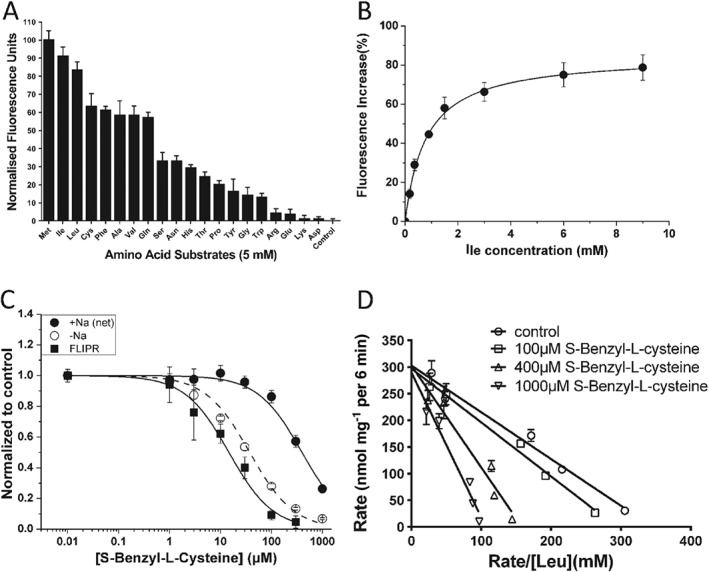
Characterization of substrate transport and substrate analogues. (A) The substrate specificity of B0AT1 expressed in CHO‐BC cells was determined using a variety of amino acids (5 mM) in the FLIPR assay. The transporter prefers large neutral amino acids and does not transport charged amino acids (n = 5). Data were normalized to the strongest signal elicited by methionine. (B) Isoleucine concentrations as indicated were used to determine its KM using the FLIPR assay (n = 5). (C) S‐Benzyl‐L‐cysteine inhibited [14C]leucine uptake (150 μM) via B0AT1 and the endogenous isoleucine transporter (n = 5). The data were compared to the action of S‐Benzyl‐L‐cysteine in the FLIPR assay. (D) The concentration dependence of [14C]leucine uptake was measured at increasing inhibitor concentrations to determine the mode of inhibition. S‐Benzyl‐L‐cysteine acts as a competitive inhibitor of leucine transport via B0AT1 (n = 5), altering the KM but not VMax. The letter ‘n’ refers to the number of independent experimental repeats for each experimental group.
