Abstract
Objective
Recently, we have shown that Bezafibrate (BEZ), the pan-PPAR (peroxisome proliferator-activated receptor) activator, ameliorated diabetes in insulin deficient streptozotocin treated diabetic mice. In order to study whether BEZ can also improve glucose metabolism in a mouse model for fatty liver and type 2 diabetes, the drug was applied to TallyHo mice.
Methods
TallyHo mice were divided into an early (ED) and late (LD) diabetes progression group and both groups were treated with 0.5% BEZ (BEZ group) or standard diet (SD group) for 8 weeks. We analyzed plasma parameters, pancreatic beta-cell morphology, and mass as well as glucose metabolism of the BEZ-treated and control mice. Furthermore, liver fat content and composition as well as hepatic gluconeogenesis and mitochondrial mass were determined.
Results
Plasma lipid and glucose levels were markedly reduced upon BEZ treatment, which was accompanied by elevated insulin sensitivity index as well as glucose tolerance, respectively. BEZ increased islet area in the pancreas. Furthermore, BEZ treatment improved energy expenditure and metabolic flexibility. In the liver, BEZ ameliorated steatosis, modified lipid composition and increased mitochondrial mass, which was accompanied by reduced hepatic gluconeogenesis.
Conclusions
Our data showed that BEZ ameliorates diabetes probably via reduced steatosis, enhanced hepatic mitochondrial mass, improved metabolic flexibility and elevated hepatic insulin sensitivity in TallyHo mice, suggesting that BEZ treatment could be beneficial for patients with NAFLD and impaired glucose metabolism.
Keywords: Bezafibrate, Glucose metabolism, Insulin resistance, Lipid metabolism, NAFLD
Abbreviations: BEZ, Bezafibrate; BG, blood glucose; ED, early onset of diabetes; EM, electron microscopy; HOMA-IR, homeostatic model assessment of insulin resistance; FA, fatty acid; LD, late onset of diabetes; NAFLD, non-alcoholic fatty liver disease; NEFA, non-esterified fatty acid; PPAR, peroxisome proliferator-activated receptor; qNMR, quantitative nuclear magnetic resonance; RER, respiratory exchange ratios; SD, standard diet; T2D, type 2 diabetes; TG, triglyceride
Highlights
-
•
Bezafibrate treatment reduced steatosis and ameliorated hepatic insulin resistance.
-
•
Bezafibrate treatment enhanced hepatic mitochondrial mass and metabolic flexibility.
-
•
Bezafibrate treatment increased beta-cell mass.
-
•
Bezafibrate treatment protected mice from developing diabetes.
-
•
Bezafibrate treatment normalized hyperglycemia in the manifest diabetic state.
1. Introduction
Bezafibrate (BEZ) is a member of the fibrate group that possesses the unique feature of activating all known peroxisome proliferator-activated receptors (PPARs, PPARα, PPARγ and PPARβ/δ) [1]. PPARs are transcription factors regulating crucial genes involved in fatty acid metabolism and insulin sensitivity [1]. BEZ was primarily used to treat patients with hyperlipidemia [2]; however, it was also shown to improve glucose metabolism in rodents [3] and humans [4]. Recently, we have shown that BEZ improves glucose metabolism and diabetes in insulin deficient streptozotocin treated mice [5]. To study whether BEZ could also ameliorate conditions associated with fatty liver and type 2 diabetes, we used the TallyHo mouse model. TallyHo mice were described in 2001 and are characterized by elevated plasma lipid levels and body weight, high fat mass, steatosis, and intermediate to severe diabetes [6], [7]. Compared to the classical monogenic diabetes models like ob/ob or db/db mice, the polygenic nature of TallyHo mice adds a clear benefit to these models by better resembling the human disease state of T2D [7]. Recent studies identified several diabetes loci (Tanidd1-4, Tabw3-4), which act in concert to promote diabetes, and male TallyHo mice show reduced peripheral glucose uptake and enlarged pancreatic islets [8]. Our data demonstrated that BEZ ameliorates impaired glucose metabolism in TallyHo mice via decreased hepatic fat content and suppressed hepatic gluconeogenesis in association with increased mitochondrial mass and elevated metabolic flexibility.
2. Materials and methods
2.1. Materials
All chemicals were purchased from Sigma–Aldrich (Germany) unless otherwise stated.
2.2. Animal studies
TallyHo mice were purchased from Jackson Laboratories and were bred in our animal facility. Only male mice were used in our study, and mice received a standard diet (SD) (R/M-H, Ssniff, Germany), which was supplemented with 0.5% (w/w) Bezafibrate (BEZ, B7273, Sigma–Aldrich) for the BEZ groups for 8 weeks. Animals were killed by isoflurane overdose, and dissected tissues were prepared as stated below. All data represent samples taken after 8 weeks of BEZ (or SD) treatment unless otherwise stated. All animals received humane care, and mouse studies were approved by local government authorities and performed according to GV-SOLAS (Society for Laboratory Animal Science) in accordance with the German Animal Welfare Act.
Plasma triglyceride (TG), non-esterified fatty acid (NEFA), glycerol, glucose, and C-reactive protein (CRP) levels were quantified using an AU480 clinical chemistry analyzer (Beckman Coulter, Germany) [9]. Blood glucose levels were measured in tail blood samples using a point of care glucometer (Contour, Bayer, Germany) and plasma insulin levels were determined using ELISA or Multi-Spot electrochemiluminescence Assay System (Mesoscale, Rockville, USA). Intraperitoneal glucose tolerance tests were performed 7 weeks after BEZ treatment with 1 g/kg glucose, and, since most of the tail blood values were higher than the upper limit of glucometer (>600 mg/dl), plasma glucose levels were determined by LabAssay Glucose Kit (Wako, Richmond, USA). Homeostatic model assessment of insulin resistance (HOMA-IR) value was calculated as: ((fasting glucose [mg/dl] × fasting insulin [μIU/ml])/405). Body composition and indirect calorimetry was studied 5 weeks after BEZ treatment as described previously [5].
2.3. Euglycemic-hyperinsulinemic clamps
Euglycemic-hyperinsulinemic clamp studies were performed 6–7 weeks after BEZ treatment as previously published [10]. To initiate the euglycemic-hyperinsulinemic clamp, a continuous insulin infusion (6 mU/kg min−1; Humulin R, Lilly, Indianapolis, USA) was started and continued for 120 min. Between 90 and 120 min, four blood samples were collected for calculation of insulin-mediated suppression of endogenous glucose appearance rates (EndoRa), a marker of hepatic glucose production. At 120 min, 2-deoxy-D-[1-14C]glucose was injected intravenously (370 kBq), and additional blood samples were collected. Basal EndoRa was calculated as the ratio of [3-3H]glucose infusion rate and plasma [3-3H]glucose specific activity. The EndoRa during insulin-stimulated conditions was determined by subtracting the Glucose Infusion Rate (GINF) from rate of disappearance (Rd). Tissue 2-[14C]deoxyglucose-6-phosphate was extracted, and glucose uptake rates (Rg) were calculated as previously described [11]. Whole body glycolysis rates were calculated from the increase in plasma 3H2O concentration, the latter referring to the difference between 3H counts before and after drying, divided by the specific activity of plasma [3-3H]glucose and the plasma 3H2O concentration.
2.4. Immunofluorescence staining
Pancreata were fixed in 4% paraformaldehyde, and cryosections were stained with anti-insulin or anti-glucagon antibodies as described previously [5].
2.5. Histochemistry
Liver tissues were fixed in 4% paraformaldehyde, and paraffin sections were stained with hematoxylin and eosin as described previously [5].
2.6. Hepatic lipid levels
Liver samples were homogenized in PBS containing 1% Triton X-100 using a TissueLyser (Qiagen, Hilden, Germany). Triglyceride (TG) levels were quantified in the homogenates using the ADVIA XPT clinical chemistry analyzer (Siemens Healthcare Diagnostics, Eschborn, Germany). Trans-esterification of the fatty acids and quantification by gas chromatography with flame ionization detection was performed as described previously [12].
2.7. Real-time PCR
Mouse livers were pulverized in liquid nitrogen and total RNA was prepared using an RNeasy Mini kit (Qiagen). cDNA was prepared by reverse transcription (Thermo Fischer Scientific, Waltham, USA), and real-time PCR assays were carried out with a LC480 Light Cycler (Roche, Mannheim, Germany) with (Scd1, Scd2, Fasn and Gapdh) or without (Cs, Ndufab1, Cox19, Cpt2, Hadha and Rps2) universal probe library (Roche). Calculations were done by a comparative method (2−ΔΔCt) and normalized to Gapdh (for Scd1, Scd2 and Fasn) or Rps2 (for Cs, Ndufab1, Cox19, Cpt2 and Hadha) as housekeeping genes. The applied primer sequences are shown in Suppl. Table 2.
2.8. Transmission electron microscopy
Liver and quadriceps samples were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer and were analyzed as described previously [13].
2.9. Western blot
Liver homogenates were loaded to an acrylamide gel and western blots were performed as described earlier [5]. α/β-tubulin antibody was purchased from Cell Signaling Technology (Cambridge, UK), citrate synthase antibody from Abcam (Cambridge, UK), and secondary antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, USA).
2.10. Statistics
Statistical evaluations were performed using GraphPad Prism 6.07. ANOVA with post hoc Holm-Šídák's multiple comparison tests were used to calculate statistical significance comparing four groups or two-tailed, unpaired Student's tests were applied with unequal distribution when two groups were compared. Statistical significance was assumed at p < 0.05.
3. Results
3.1. TallyHo mice
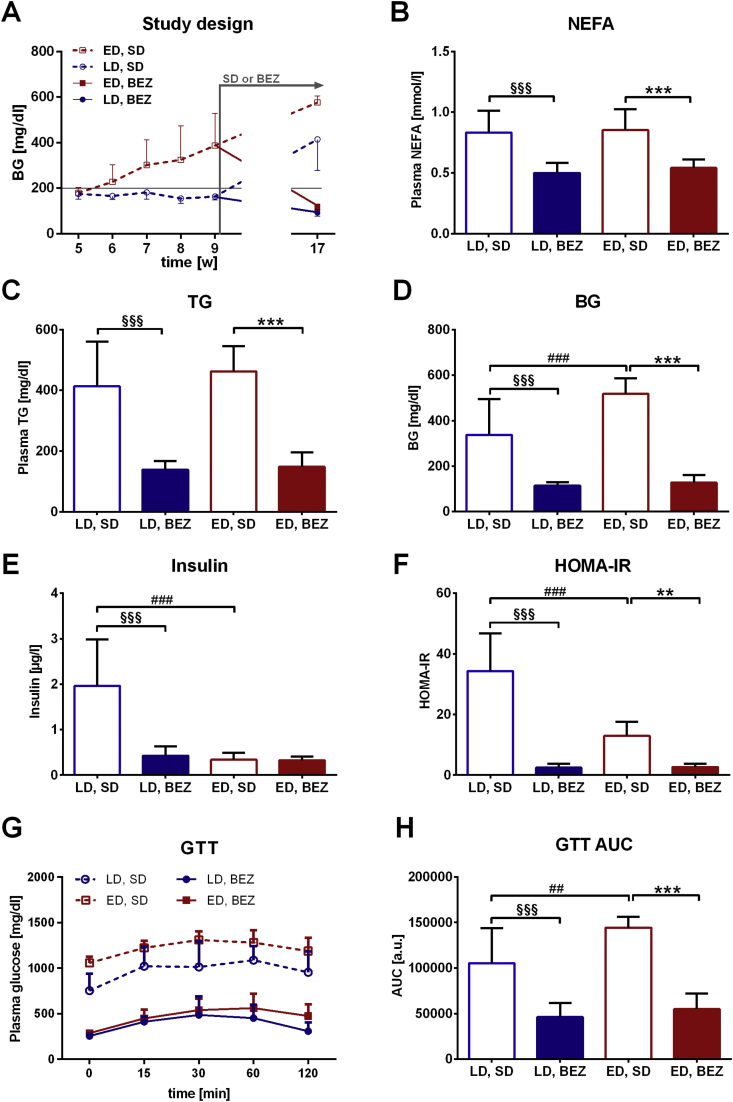
TallyHo mice represent a polygenic mouse model for diabetes with mild steatosis and insulin resistance. However, the mice display individual onset of diabetes on standard diet; thus fast and slow progressors can be identified among them. In order to study the effect of Bezafibrate (BEZ) in different stages of diabetes, TallyHo mice were divided into two groups at 9 weeks of age. Mice with fasting blood glucose <200 mg/dl were defined as late onset of diabetes (LD) group, whereas mice with blood glucose values > 200 mg/dl were classified as early onset of diabetes (ED) group (Figure 1A). Thus, LD mice represented a preventive group, in which we studied whether BEZ could protect mice in a prediabetic stage from diabetes progression; whereas ED mice served as a therapeutic group, in which we investigated whether BEZ treatment could revert established diabetes.
Figure 1.
Levels of blood glucose, plasma lipid, insulin, and glucose tolerance test. A. The figure represents the averages of weekly measured blood glucose values (values for week 9 are also showed as Suppl. Figure 1A). TallyHo mice were split into two groups according to the 9 weeks old blood glucose values (early onset of diabetes (ED) group: >200 mg/dl, late onset of diabetes (LD) group: <200 mg/dl). BEZ (or SD) feeding was started at 9 weeks of age and lasted for 8 weeks. Standard diet (SD), BEZ diet (BEZ). B. Plasma non-esterified fatty acids (NEFA) and C. triglyceride (TG) levels. D. Fasted blood glucose (BG) values. E. Plasma insulin levels. F. Homeostatic model assessment of insulin resistance (HOMA-IR) values. G. Glucose tolerance test (GTT) and H. area under the curve (AUC) evaluation. Columns represent averages ± standard deviations; n = 6–12. *denotes significant differences between ED, BEZ vs. ED, SD; **p < 0.01, ***p < 0.001; #denotes significant differences between ED, SD vs. LD, SD; ##p < 0.01, ###p < 0.001; §denotes significant differences between LD, BEZ vs. LD, SD; §§§p < 0.001.
3.2. BEZ ameliorates diabetes, reduces plasma lipid levels, and improves glucose tolerance
In order to study whether BEZ has a beneficial effect to prevent the development of (LD group) or ameliorate T2D (ED group), both TallyHo groups were treated for 8 weeks with the BEZ containing diet (BEZ group) or with standard diet (SD). At 9 weeks of age, ED, SD mice already showed higher BG levels compared to LD, SD mice (Suppl. Figure 1A), although both untreated groups developed diabetes by the age of 17 weeks (Figure 1D). Compared to the SD groups, BEZ reduced plasma lipid and glycerol levels (Figure 1B,C and Suppl. Figure 1B), suggesting reduced lipolysis. Furthermore, the level of CRP, which is an inflammatory marker, tended to decrease upon BEZ treatment (Suppl. Figure 1C), indicating a possible amelioration of inflammatory processes. BEZ markedly decreased blood glucose levels measured by a glucometer (Figure 1D), which was also verified by plasma glucose measurements using glucose assay (Suppl. Figure 1D). Insulin levels were lower (LD, BEZ vs LD, SD) or remained unchanged (ED, BEZ vs ED, SD) in the BEZ groups (Figure 1E). As a consequence, HOMA-IR values were normalized in both BEZ groups (Figure 1F). Furthermore, BEZ attenuated the impaired glucose tolerance of TallyHo mice (Figure 1G,H) without increasing insulin levels during the glucose tolerance test (Suppl. Figure 1E–F). These results suggest that BEZ improved insulin sensitivity and glucose metabolism, which in turn resulted in the normalization of blood glucose levels independently of plasma insulin levels.
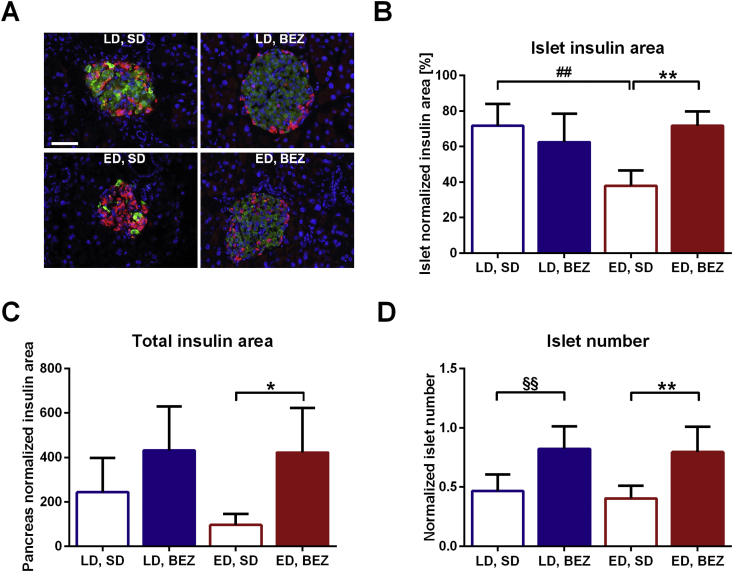
3.3. BEZ increases beta-cell mass
Next, we studied the pancreatic architecture of BEZ-treated animals. BEZ elevated the content of insulin producing beta-cells in the islets (Figure 2A–B) as it also increased total insulin area in the pancreas as well as total islet number (Figure 2C,D). Glucagon area normalized to islets was higher in the ED, SD group compared to LD, SD mice, whereas total glucagon area remained unchanged (Suppl. Figure 2).
Figure 2.
Pancreas architecture. A. Pancreata were stained with anti-insulin (green) and anti-glucagon (red) antibodies and visualized by fluorescence microscopy. Cell nuclei were stained with DAPI (blue). The white bar represents 50 μm. Representative areas are shown. B. Insulin area normalized to islet area and C. total insulin area normalized to pancreas area were calculated using Architect software. D. Islet number was manually counted and values were normalized to total pancreas area. Columns represent averages ± standard deviations; n = 5. *denotes significant differences between ED, BEZ vs. ED, SD; *p < 0.05, **p < 0.01; #denotes significant differences between ED, SD vs. LD, SD; ##p < 0.01; §denotes significant differences between LD, BEZ vs. LD, SD; §§p < 0.01.
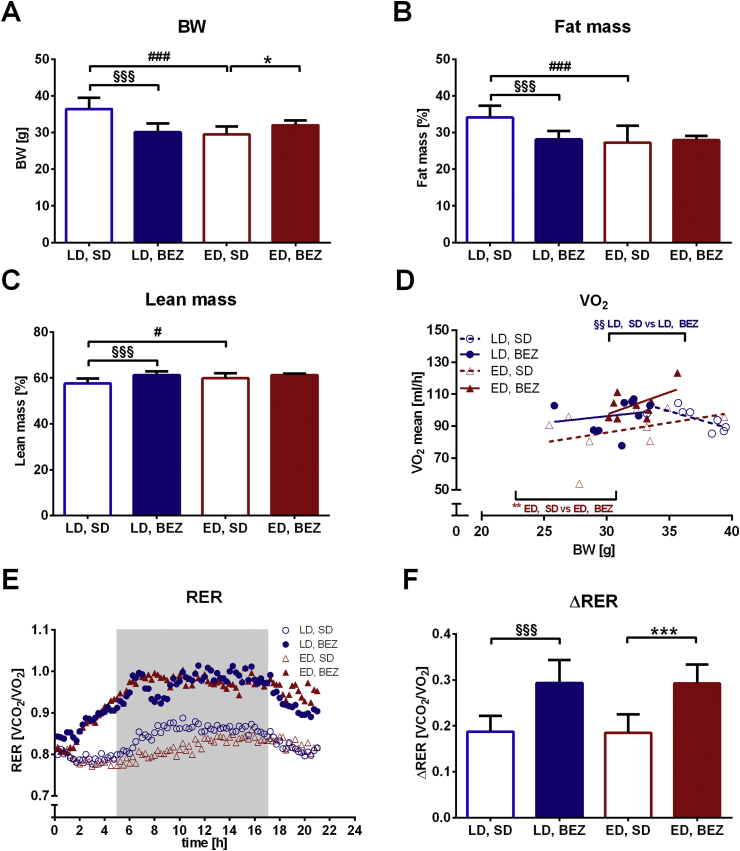
3.4. BEZ elevates energy expenditure and metabolic flexibility
BEZ treatment resulted in lower body weight in the LD group; however, there was an opposite effect in ED group (Figure 3A). BEZ increased relative lean and decreased fat mass in LD mice (Figure 3B–C and Suppl. Figure 3A,B), the latter remained significant, when normalized to body weight (Suppl. Figure 3C,D). BEZ increased food consumption in LD group, but water intake remained unaltered upon drug treatment (Suppl. Figure 3E,F). BEZ elevated carbon dioxide production and oxygen consumption (Suppl. Figure 4A–D); the latter remained significant when normalized to body weight (Figure 3D) and used as a marker for energy expenditure. Respiratory exchange ratio (RER) and metabolic flexibility assessed as delta RER were also higher in BEZ-treated animals (Figure 3E–F). Rearing and run distance were not altered upon BEZ application (Suppl. Figure 4E–H). These data suggest that improved metabolic flexibility and energy expenditure could be involved in the beneficial role of BEZ.
Figure 3.
Body composition and indirect calorimetry. A. Body weight. B. Fat and C. lean mass were measured by qNMR (Suppl. Figure 3A,B) and normalized to body weights in %. D. Average oxygen consumption normalized to body weights. E. Respiratory exchange ratios (RERs) were calculated by dividing carbon dioxide production (VCO2) by oxygen consumption (VO2) (Suppl. Figure 4A–D). The gray rectangle represents 12-h dark phase (0-time point represents 1 p.m.). F. ΔRER was calculated as RERmax − RERmin. Columns represent averages ± standard deviations; n = 8–12. *denotes significant differences between ED, BEZ vs. ED, SD; *p < 0.05, **p < 0.01, ***p < 0.001; #denotes significant differences between ED, SD vs. LD, SD; #p < 0.05, ###p < 0.001; §denotes significant differences between LD, BEZ vs. LD, SD; §§p < 0.01, §§§p < 0.001.
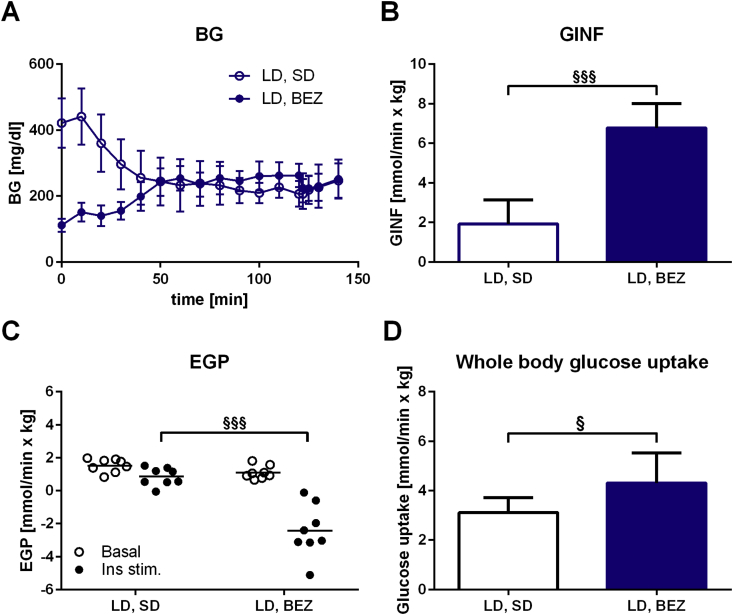
3.5. BEZ reduces hepatic gluconeogenesis in LD mice
In order to assess whether BEZ improves hepatic insulin sensitivity, we performed euglycemic-hyperinsulinemic clamps in LD mice. To reach normoglycemia at the applied insulin dose (Figure 4A), a higher glucose infusion rate was needed in the BEZ group (Figure 4B), suggesting elevated insulin sensitivity. Endogenous glucose production (EGP), which mainly consists of hepatic gluconeogenesis [14], was inhibited in both groups with a significantly stronger effect in the BEZ group (Figure 4C). Glucose flux analysis showed an elevated whole body glucose uptake in the BEZ group (Figure 4D), however glucose uptake was not increased in M. quadriceps or epididymal fat tissues (Suppl. Figure 5A). Glucosuria was normalized under BEZ treatment, suggesting the absence of diabetic urinary glucose loss (Suppl. Figure 5B). These results suggest that BEZ improved the hepatic insulin sensitivity via reduced gluconeogenesis, which is involved in the amelioration of diabetes.
Figure 4.
Euglycemic-hyperinsulinemic clamp. A. Steady state BG levels during the clamp. B. Glucose infusion rate (GINF). C. Endogenous glucose production (EGP). D. Whole body glucose uptake. Columns represent averages ± standard deviations; n = 8 animals. §denotes significant differences between LD, BEZ vs. LD, SD; §p < 0.05, §§§p < 0.001.
3.6. BEZ reduces hepatic lipid contents in LD mice and elevates relative MUFA contents in ED mice
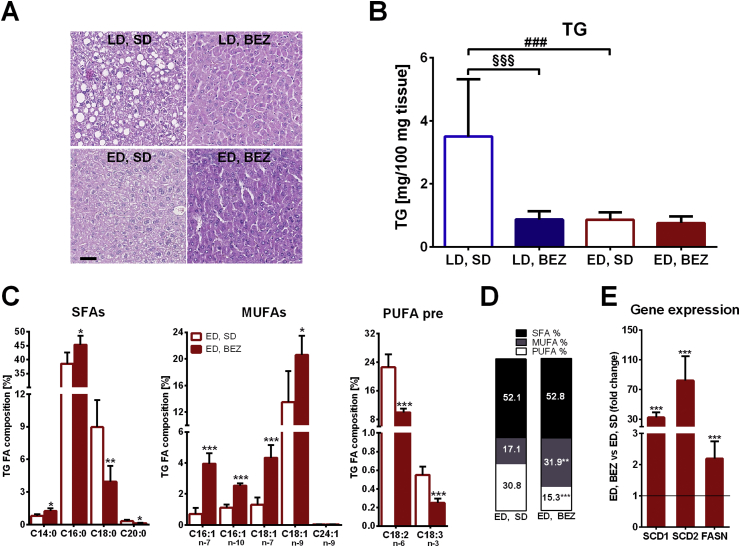
To study the underlying mechanisms of the improved hepatic gluconeogenesis, the hepatic lipid content of BEZ-treated mice was assessed. Histological staining showed reduced steatosis in LD mice upon BEZ treatment (Figure 5A), which was associated with reduced total hepatic TG content (Figure 5B). Since fatty liver is a key component of the metabolic syndrome and strongly associated with insulin resistance [15], these data suggest that BEZ improves insulin sensitivity in LD TallyHo mice possibly through reduced hepatic steatosis. Lipotoxic effects of FA and lipid intermediates counteract insulin signaling, and these effects are implicated in the pathogenesis of fatty liver and insulin resistance. Since chain lengths and saturation state have major impact on lipotoxic actions of FA, and monounsaturated FAs (MUFAs) have beneficial effects on patients with T2D [16], we determined the exact FA composition of hepatic triglycerides. As expected from the reduced total hepatic TG content (Figure 5B), most of the TGs (normalized to liver weight) were lower in LD, BEZ animals compared to LD, SD mice; ED, SD mice also showed lower TGs compared to LD, SD group (Suppl. Table 1). The total TG content is known to influence the relative composition of TGs with various FA lengths and saturation state [12]. Therefore, we compared the relative FA composition only between ED, BEZ and ED, SD groups, in which the total TG contents were comparable (Figure 5B). BEZ increased the relative content of C14:0 and C16:0 FAs and decreased C18:0 and C20:0 FA contents (Figure 5C, left panel). C16:1 and C18:1 MUFAs were markedly increased upon BEZ treatment (Figure 5C, middle panel); however, the precursor n-3 and n-6 polyunsaturated FAs (PUFAs) as well as many other PUFAs were decreased (Figure 5C, right panel and Suppl. Figure 5C,D). BEZ elevated total MUFA but reduced total PUFA content (Figure 5D). More importantly compared to the ED, SD group, ED, BEZ mice showed higher MUFA/SFA ratio (0.614 ± 0.176 vs 0.340 ± 0.139, p = 0.0121), elevated stearoyl-CoA-desaturase (SCD) activity index (cis-C16:1n-7/C16:0; 0.088 ± 0.032 vs 0.019 ± 0.012, p = 0.0003), and increased de-novo lipogenesis index (C16:0/C18:2n-6; 4.66 ± 1.95 vs 1.76 ± 0.42, p = 0.0011). In order to investigate the role of SCDs and FA synthesis the transcript levels of Scd1 and 2 as well as the fatty acid synthase (FASN) were studied by real-time PCR. BEZ elevated the mRNA level of both SCDs and FASN (Figure 5E). These results suggest that BEZ increases hepatic lipogenesis and SCD activity, which, in turn, elevates the content of MUFAs. On the other hand, the reduced PUFA precursors (C18:3n-3 and C18:2n-6) and PUFAs suggest that BEZ also elevates FA oxidation.
Figure 5.
Hepatic lipid content. A. Hematoxylin and eosin staining of the liver, the black bar represents 50 μm. Representative areas are shown. B. Liver total TG levels and C. relative liver TG fatty acid (FA) composition. n – “number” denotes the position of double bounds counted from the omega carbon. Saturated FA (SFA), monounsaturated FA (MUFA) and polyunsaturated FA (PUFA), pre: precursor. D. The relative content of total SFA, MUFA and PUFA in TG fraction denoted as % of total FA. E. ED, SD group normalized relative mRNA levels of the indicated transcripts. Scd: Stearoyl-CoA-desaturase, Fasn: fatty acid synthase. Columns represent averages ± standard deviations; A, C, D and E represent n = 4–7; B represents n = 8–9 animals. *denotes significant differences between ED, BEZ vs. ED, D; *p < 0.05, **p < 0.01, ***p < 0.001. #denotes significant differences between ED, SD vs. LD, SD; ###p < 0.001; §denotes significant differences between LD, BEZ vs. LD, SD; §§§p < 0.001.
3.7. BEZ increases mitochondrial mass in TallyHo mice
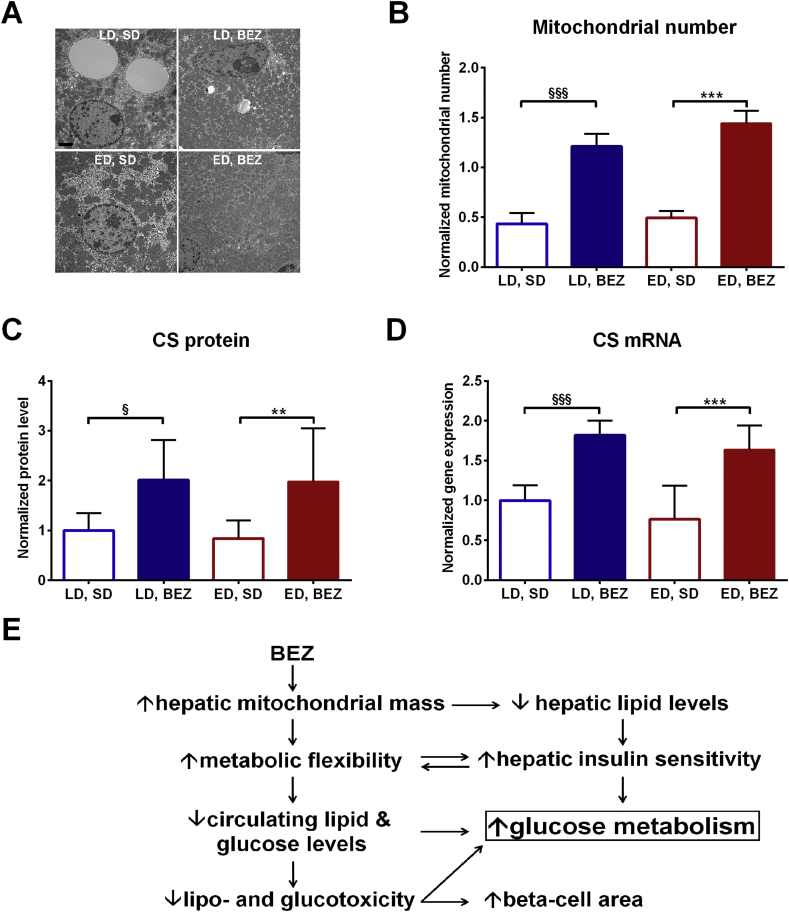
Since mitochondria play a crucial role in FA oxidation, we studied mitochondrial mass using different approaches. Transmission EM revealed a higher mitochondrial number in the BEZ groups compared to untreated controls (Figure 6A,B). Citrate synthase (CS) is usually used as a marker for mitochondrial mass, and protein as well as mRNA level of CS were elevated in BEZ-treated animals (Figure 6C,D and Suppl. Figure 6A). Higher transcript levels of other mitochondrial genes were also found (Suppl. Figure 6B–E). These results indicate that BEZ induces mitochondrial biogenesis in the liver of TallyHo mice. The elevated mitochondrial mass and decreased lipid and PUFA precursor content in the liver of LD, BEZ mice suggest increased hepatic fatty acid oxidation, which could be involved in the attenuated hepatic gluconeogenesis. Moreover, BEZ increased hepatic MUFA contents, which is possibly involved in the amelioration of glucose metabolism in ED, BEZ group. Skeletal muscle of BEZ-treated animals showed normal mitochondrial mass (Suppl. Figure 7).
Figure 6.
Hepatic mitochondrial mass. A. Liver mitochondrial mass and architecture were assessed by transmission EM, the black bar denotes 2 μm. Representative areas are shown. B. Mitochondrial number was quantified in five independent regions and normalized to the analyzed area (μm2). C. Hepatic citrate synthase (CS) protein level was analyzed using western blot, and the intensity of the bands was normalized to tubulin and depicted as ratio to LD, SD group. Representative pictures are shown in Suppl. Figure 6A. D. Hepatic gene expression was studied using real-time PCR and depicted as ratio to LD, SD group. CS: citrate synthase E. Our data demonstrated that BEZ improves glucose metabolism in TallyHo mice. In this scheme, the possible underlying mechanisms observed in LD mice are depicted, which are probably involved in the beneficial effects of BEZ. Columns represent averages ± standard deviations; A–B represent n = 4; C–D represent n = 7–9 animals. *denotes significant differences between ED, BEZ vs. ED, SD; **p < 0.01, ***p < 0.001; §denotes significant differences between LD, BEZ vs. LD, SD; §p < 0.05, §§§p < 0.001.
4. Discussion
The major finding of our study was that BEZ-treated, slow progressor (late onset of diabetes) LD TallyHo mice were protected against diabetes, but, more importantly, the established diabetes in early onset of diabetes (ED) group was reverted upon drug application. The anti-diabetic potential of BEZ was also reported in rodent models for T1D [5] and T2D [3], [17] as well as in patients with T2D [4], [18], [19]. Furthermore, diabetes prevalence in patients with coronary artery disease, who showed impaired glucose tolerance, was also attenuated after BEZ application [20]. These results indicate that BEZ could indeed prevent the progression of a prediabetic state to clinical diabetes and even revert an established diabetic state in rodents and humans; however, the underlying mechanisms are currently poorly understood.
The anti-diabetic effect of the drug is probably attributed to its insulin sensitizing capacity indicated by stronger inhibition of endogenous glucose production and decreased HOMA-IR index. Tenenbaum et al. also reported that BEZ treatment was indeed efficient to prevent the increase of HOMA-IR index during two year follow up in patients with coronary artery disease [21]. Although fenofibrate did not change HOMA-IR in patients with impaired glucose tolerance or diabetes, BEZ did significantly decrease HOMA-IR compared to placebo group after 8 weeks of treatment [22] as other studies also reported lower HOMA-IR in BEZ-treated patients [19], [23].
In our euglycemic-hyperinsulinemic clamp experiments, insulin stimulus of BEZ-treated animals caused negative endogenous glucose production (EGP) values. Negative or “zero” values for EGP were also reported in rodent models by us [10] and others [14], [24], [25], [26] and could be attributed to the high glucose infusion rate [27] in the BEZ group due to the big difference in insulin sensitivity between BEZ and SD groups. Therefore, the negative values of the BEZ-treated mice are assumed to correspond to “zero” as also reported by others [14]. Despite the negative EGP values, our results clearly showed that BEZ treatment led to a pronounced reduction in EGP reflecting improved hepatic insulin sensitivity. There are only a few studies reporting insulin sensitivity from euglycemic-hyperinsulinemic clamp experiments in patients, and BEZ treatment showed no alteration in insulin sensitivity in patients with high lipid levels [28] or diabetes [29], while others found an improved insulin sensitivity [30], [31]. These results indicate that further studies are needed to investigate the role of BEZ in insulin sensitivity in human subjects. However, dual PPARα and PPARδ activation was recently shown to improve hepatic insulin sensitivity in patients with insulin resistance [32].
In contrast to the low insulin levels of BEZ-treated mice, these animals showed an increased beta-cell area compared to untreated controls. BEZ has been shown to improve islet architecture in diabetic mice with type 1 diabetes [5], [33], and, compared to the PPARα activator fenofibrate or PPARγ activator rosiglitazone, it was the only PPAR activator, which prevented the compensatory islet hypertrophy in high sucrose, high fat diet treated mice [34], pointing to its unique attribute. PPAR activation could directly improve beta-cell function [35] or could also occur as a secondary consequence of the amelioration of gluco- and lipotoxicity.
Metabolic flexibility is assumed as the ability to change substrate oxidation from fat to carbohydrate and its malfunction is intimately related to insulin resistance and ectopic lipid accumulation [36]. Since mitochondria play a crucial role in substrate oxidation [37], mitochondrial dysfunction was reported in skeletal muscle of insulin resistant mouse models [38] and patients [39] as well as in patients with type 2 diabetes, and it was associated with metabolic inflexibility [40]. Furthermore, impaired mitochondrial beta-oxidation was postulated to contribute to hepatic steatosis [41]. Therefore, the elevated mitochondrial mass found in the liver of BEZ-treated animals could be involved in the improved metabolic flexibility. In TallyHo mice, BEZ elevated respiratory exchange ratio (RER), which reflects higher carbohydrate oxidation, and the higher delta RER upon BEZ treatment indicates better metabolic flexibility. BEZ treatment of patients with PNPLA2 mutation indeed improved metabolic flexibility, which was associated with better insulin sensitivity [31], suggesting an overall effect of BEZ in mice and humans. In addition, the enhanced energy expenditure observed in BEZ-treated TallyHo mice is postulated to be beneficial in the prevention of lipid accumulation and insulin resistance [42].
Compared to LD, SD animals, ED, SD mice exhibited decreased hepatic fat content, which is probably attributed to the long lasting diabetic and insulin deficient state since the diminished insulin level could impair fat storage. On the other hand, LD, SD mice are a good model for non-alcoholic fatty liver disease (NAFLD), since they showed hepatic steatosis and insulin resistance, which are hallmarks for NAFLD [43]. The precursors of PUFAs, which cannot be endogenously synthesized but only supplied by the food, showed lower hepatic contents in BEZ-treated animals in association with reduced content of other PUFAs. These data indicate the BEZ elevated FA oxidation and as a consequence decreased hepatic lipid levels. The lower hepatic TG level and the increased mitochondrial mass observed in the BEZ-treated LD TallyHo mice suggest an improved FA metabolism, which could lead to less lipid intermediates attenuating insulin resistance and enhancing the inhibitory effect of insulin on endogenous glucose production (Figure 6E). In addition to reducing lipid levels in LD mice, BEZ also changed the fatty acid composition of ED mice. PPARα knock-out mice are characterized by lower C16:1n-7 fatty acid level in hepatic TG fraction compared to wild-type controls [44]. Thus, the 5.7-times higher C16:1n-7 level upon BEZ treatment in ED mice suggests that PPARα plays an important role in elevating MUFAs. A diet enriched in MUFAs was shown to significantly decrease HbA1c, plasma glucose levels, and HOMA-IR index in patients with T2D [16]. Thus, the elevated hepatic MUFAs in the BEZ-treated animals could also participate in ameliorating insulin sensitivity and diabetes. Stearoyl-CoA-desaturase (SCD) is the corresponding enzyme, which is responsible for the production of C16:1n-7 and C18:1n-9. In BEZ-treated ED animals, higher mRNA levels of both SCD isoform and elevated SCD activity index were observed. Since high hepatic SCD1 activity was associated with low hepatic fat content and insulin sensitivity in human subjects [45], [46], [47], an increased SCD activity in BEZ-treated mice could contribute to the improved hepatic insulin sensitivity.
Although the combined treatment of NAFLD, obesity, and T2D is intensively studied, currently there are only limited drugs available [48]. In the recent years, novel dual PPARα and PPARγ agonists were studied; however, most of the new candidates showed undesirable effects [49]. In contrast to them, BEZ is already 40 years on the market, it activates all three PPARs, and it has a good safety [20]. Recently, a novel PPARα and PPARδ activator, Elafibranor (GFT505) was shown to have beneficial effects improving endogenous glucose production, blood glucose levels, and steatosis in patients with insulin resistance or NASH, respectively [32], [50]. These observations and our data suggest that the activation of PPARs may represent a good treatment option for subjects with diabetes and NAFLD.
Acknowledgements
This work has been funded by the German Federal Ministry of Education and Research to the German Mouse Clinic (Infrafrontier grant 01KX1012) and the German Center for Diabetes Research (DZD e.V.). We thank Michael Schulz, Moya Wu, Anett Seelig, and Jürgen Schultheiβ for excellent technical assistance.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.12.007.
Contributor Information
Andras Franko, Email: andras.franko@med.uni-tuebingen.de.
Martin Hrabě de Angelis, Email: hrabe@helmholtz-muenchen.de.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutrition Journal. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohno Y., Miyoshi T., Noda Y., Oe H., Toh N., Nakamura K. Bezafibrate improves postprandial hypertriglyceridemia and associated endothelial dysfunction in patients with metabolic syndrome: a randomized crossover study. Cardiovascular Diabetology. 2014;13:71. doi: 10.1186/1475-2840-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui H., Okumura K., Kawakami K., Hibino M., Toki Y., Ito T. Improved insulin sensitivity by bezafibrate in rats: relationship to fatty acid composition of skeletal-muscle triglycerides. Diabetes. 1997;46:348–353. doi: 10.2337/diab.46.3.348. [DOI] [PubMed] [Google Scholar]
- 4.Jones I.R., Swai A., Taylor R., Miller M., Laker M.F., Alberti K.G. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care. 1990;13:855–863. doi: 10.2337/diacare.13.8.855. [DOI] [PubMed] [Google Scholar]
- 5.Franko A., Huypens P., Neschen S., Irmler M., Rozman J., Rathkolb B. Bezafibrate improves insulin sensitivity and metabolic flexibility in STZ-induced diabetic mice. Diabetes. 2016;65:2540–2552. doi: 10.2337/db15-1670. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.H., Sen S., Avery C.S., Simpson E., Chandler P., Nishina P.M. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74:273–286. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- 7.Leiter E.H., Strobel M., O'Neill A., Schultz D., Schile A., Reifsnyder P.C. Comparison of two new mouse models of polygenic type 2 diabetes at the Jackson Laboratory, NONcNZO10Lt/J and TALLYHO/JngJ. Journal of Diabetes Research. 2013;2013:165327. doi: 10.1155/2013/165327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.H., Saxton A.M. The TALLYHO mouse as a model of human type 2 diabetes. Methods in Molecular Biology. 2012;933:75–87. doi: 10.1007/978-1-62703-068-7_6. [DOI] [PubMed] [Google Scholar]
- 9.Rathkolb B., Hans W., Prehn C., Fuchs H., Gailus-Durner V., Aigner B. Clinical chemistry and other laboratory tests on mouse plasma or serum. Current Protocols in Mouse Biology. 2013;3:69–100. doi: 10.1002/9780470942390.mo130043. [DOI] [PubMed] [Google Scholar]
- 10.Franko A., von Kleist-Retzow J.C., Neschen S., Wu M., Schommers P., Bose M. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. Journal of Hepatology. 2014;60:816–823. doi: 10.1016/j.jhep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Konstantopoulos N., Molero J.C., McGee S.L., Spolding B., Connor T., de Vries M. Methazolamide is a new hepatic insulin sensitizer that lowers blood glucose in vivo. Diabetes. 2012;61:2146–2154. doi: 10.2337/db11-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter A., Kovarova M., Nadalin S., Cermak T., Konigsrainer A., Machicao F. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia. 2014;57:2103–2107. doi: 10.1007/s00125-014-3310-0. [DOI] [PubMed] [Google Scholar]
- 13.Franko A., Baris O.R., Bergschneider E., von Toerne C., Hauck S.M., Aichler M. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PLoS One. 2013;8:e82392. doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choukem S.P., Gautier J.F. How to measure hepatic insulin resistance? Diabetes & Metabolism. 2008;34:664–673. doi: 10.1016/S1262-3636(08)74602-0. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N., Haring H.U. The role of hepatokines in metabolism. Nature Reviews Endocrinology. 2013;9:144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 16.Schwingshackl L., Strasser B. High-MUFA diets reduce fasting glucose in patients with type 2 diabetes. Annals of Nutrition and Metabolism. 2012;60:33–34. doi: 10.1159/000335162. [DOI] [PubMed] [Google Scholar]
- 17.Jia D., Yamamoto M., Otani M., Otsuki M. Bezafibrate on lipids and glucose metabolism in obese diabetic Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2004;53:405–413. doi: 10.1016/j.metabol.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Wahl P., Hasslacher C., Lang P.D., Vollmar J. [Lipid-lowering effect of bezafibrate in patients with diabetes mellitus and hyperlipidaemia (author's transl)] Deutsche Medizinische Wochenschrift. 1978;103:1233–1237. doi: 10.1055/s-0028-1129237. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima M., Taniguchi A., Sakai M., Doi K., Nagata I., Nagasaka S. Effect of bezafibrate on insulin sensitivity in nonobese Japanese type 2 diabetic patients. Diabetes Care. 2000;23:259. doi: 10.2337/diacare.23.2.259. [DOI] [PubMed] [Google Scholar]
- 20.Tenenbaum A., Motro M., Fisman E.Z., Schwammenthal E., Adler Y., Goldenberg I. Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation. 2004;109:2197–2202. doi: 10.1161/01.CIR.0000126824.12785.B6. [DOI] [PubMed] [Google Scholar]
- 21.Tenenbaum A., Fisman E.Z., Boyko V., Benderly M., Tanne D., Haim M. Attenuation of progression of insulin resistance in patients with coronary artery disease by bezafibrate. Archives of Internal Medicine. 2006;166:737–741. doi: 10.1001/archinte.166.7.737. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi T., Kobayashi J., Yagi K., Nohara A., Yamaaki N., Sugihara M. Comparison of effects of bezafibrate and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 and adipocytokine levels in dyslipidemic subjects with impaired glucose tolerance or type 2 diabetes mellitus: results from a crossover study. Atherosclerosis. 2011;217:165–170. doi: 10.1016/j.atherosclerosis.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Jonkers I.J., Mohrschladt M.F., Westendorp R.G., van der Laarse A., Smelt A.H. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: reversal with bezafibrate therapy in a randomized controlled trial. American Journal of Medicine. 2002;112:275–280. doi: 10.1016/s0002-9343(01)01123-8. [DOI] [PubMed] [Google Scholar]
- 24.Bonner J.S., Lantier L., Hocking K.M., Kang L., Owolabi M., James F.D. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes. 2013;62:3251–3260. doi: 10.2337/db13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H.Y., Ducommun S., Quan C., Xie B., Li M., Wasserman D.H. AS160 deficiency causes whole-body insulin resistance via composite effects in multiple tissues. Biochemical Journal. 2013;449:479–489. doi: 10.1042/BJ20120702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brand C.L., Sturis J., Gotfredsen C.F., Fleckner J., Fledelius C., Hansen B.F. Dual PPARalpha/gamma activation provides enhanced improvement of insulin sensitivity and glycemic control in ZDF rats. American Journal of Physiology Endocrinology and Metabolism. 2003;284:E841–E854. doi: 10.1152/ajpendo.00348.2002. [DOI] [PubMed] [Google Scholar]
- 27.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Disease Models & Mechanisms. 2010;3:525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karhapaa P., Uusitupa M., Voutilainen E., Laakso M. Effects of bezafibrate on insulin sensitivity and glucose tolerance in subjects with combined hyperlipidemia. Clinical Pharmacology and Therapeutics. 1992;52:620–626. doi: 10.1038/clpt.1992.200. [DOI] [PubMed] [Google Scholar]
- 29.Riccardi G., Genovese S., Saldalamacchia G., Patti L., Marotta G., Postiglione A. Effects of bezafibrate on insulin secretion and peripheral insulin sensitivity in hyperlipidemic patients with and without diabetes. Atherosclerosis. 1989;75:175–181. doi: 10.1016/0021-9150(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 30.Shiochi H., Ohkura T., Fujioka Y., Sumi K., Yamamoto N., Nakanishi R. Bezafibrate improves insulin resistance evaluated using the glucose clamp technique in patients with type 2 diabetes mellitus: a small-scale clinical study. Diabetology & Metabolic Syndrome. 2014;6:113. doi: 10.1186/1758-5996-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Weijer T., Havekes B., Bilet L., Hoeks J., Sparks L., Bosma M. Effects of bezafibrate treatment in a patient and a carrier with mutations in the PNPLA2 gene, causing neutral lipid storage disease with myopathy. Circulation Research. 2013;112:e51–54. doi: 10.1161/CIRCRESAHA.113.300944. [DOI] [PubMed] [Google Scholar]
- 32.Cariou B., Hanf R., Lambert-Porcheron S., Zair Y., Sauvinet V., Noel B. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anwer T., Sharma M., Pillai K.K., Haque S.E., Alam M.M., Zaman M.S. Protective effect of bezafibrate on streptozotocin-induced oxidative stress and toxicity in rats. Toxicology. 2007;229:165–172. doi: 10.1016/j.tox.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes-Santos C., Evangelista Carneiro R., de Souza Mendonca L., Barbosa Aguila M., Mandarim-de-Lacerda C.A. Rosiglitazone aggravates nonalcoholic fatty pancreatic disease in C57BL/6 mice fed high-fat and high-sucrose diet. Pancreas. 2009;38:e80–86. doi: 10.1097/MPA.0b013e3181987d9d. [DOI] [PubMed] [Google Scholar]
- 35.Cavaghan M.K., Ehrmann D.A., Byrne M.M., Polonsky K.S. Treatment with the oral antidiabetic agent troglitazone improves beta cell responses to glucose in subjects with impaired glucose tolerance. Journal of Clinical Investigation. 1997;100:530–537. doi: 10.1172/JCI119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galgani J.E., Moro C., Ravussin E. Metabolic flexibility and insulin resistance. American Journal of Physiology, Endocrinology and Metabolism. 2008;295:E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franko A., Hrabe De Angelis M., Wiesner R.J. Mitochondrial function, dysfunction and adaptation in the liver during the development of diabetes. In: Han D., Kaplowitz N., editors. Mitochondria in liver disease. CRC Press; Abingdon, UK: 2015. pp. 383–411. [Google Scholar]
- 38.Franko A., von Kleist-Retzow J.C., Bose M., Sanchez-Lasheras C., Brodesser S., Krut O. Complete failure of insulin-transmitted signaling, but not obesity-induced insulin resistance, impairs respiratory chain function in muscle. Journal of Molecular Medicine. 2012;90:1145–1160. doi: 10.1007/s00109-012-0887-y. [DOI] [PubMed] [Google Scholar]
- 39.Sleigh A., Raymond-Barker P., Thackray K., Porter D., Hatunic M., Vottero A. Mitochondrial dysfunction in patients with primary congenital insulin resistance. Journal of Clinical Investigation. 2011;121:2457–2461. doi: 10.1172/JCI46405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Weijer T., Sparks L.M., Phielix E., Meex R.C., van Herpen N.A., Hesselink M.K. Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One. 2013;8:e51648. doi: 10.1371/journal.pone.0051648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardwick J.P., Osei-Hyiaman D., Wiland H., Abdelmegeed M.A., Song B.J. PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-hydroxylase (CYP4) isozymes: implications for prevention of lipotoxicity in fatty liver disease. PPAR Research. 2009;2009:952734. doi: 10.1155/2009/952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel V.T., Shulman G.I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefan N., Fritsche A., Schick F., Haring H.U. Phenotypes of prediabetes and stratification of cardiometabolic risk. The Lancet Diabetes & Endocrinology. 2016;4:789–798. doi: 10.1016/S2213-8587(16)00082-6. [DOI] [PubMed] [Google Scholar]
- 44.Martin P.G., Guillou H., Lasserre F., Dejean S., Lan A., Pascussi J.M. Novel aspects of PPARalpha-mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology. 2007;45:767–777. doi: 10.1002/hep.21510. [DOI] [PubMed] [Google Scholar]
- 45.Stefan N., Peter A., Cegan A., Staiger H., Machann J., Schick F. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51:648–656. doi: 10.1007/s00125-008-0938-7. [DOI] [PubMed] [Google Scholar]
- 46.Peter A., Weigert C., Staiger H., Machicao F., Schick F., Machann J. Individual stearoyl-coa desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009;58:1757–1765. doi: 10.2337/db09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silbernagel G., Kovarova M., Cegan A., Machann J., Schick F., Lehmann R. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. Journal of Clinical Endocrinology & Metabolism. 2012;97:E2288–E2292. doi: 10.1210/jc.2012-2152. [DOI] [PubMed] [Google Scholar]
- 48.Roden M. The liver in focus. Diabetologia. 2016;59:1095–1097. doi: 10.1007/s00125-016-3911-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenson R.S., Wright R.S., Farkouh M., Plutzky J. Modulating peroxisome proliferator-activated receptors for therapeutic benefit? Biology, clinical experience, and future prospects. American Heart Journal. 2012;164:672–680. doi: 10.1016/j.ahj.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150 doi: 10.1053/j.gastro.2016.01.038. 1147–1159.e1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.