Abstract
Photosynthetic organisms have to tolerate rapid changes in light intensity, which is facilitated by non-photochemical quenching (NPQ) and involves modification of energy transfer from light-harvesting complexes (LHC) to the photosystem reaction centres. NPQ includes dissipating excess light energy to heat (qE) and the reversible coupling of LHCII to photosystems (state transitions/qT), which are considered separate NPQ mechanisms. In the model alga Chlamydomonas reinhardtii the LHCSR3 protein has a well characterised role in qE. Here, it is shown in the npq4 mutant, deficient in LHCSR3, that energy coupling to photosystem II (PSII) more akin to qT is also disrupted, but no major differences in LHC phosphorylation or LHC compositions were found in comparison to wild-type cells. The qT of wild-type cells possessed two kinetically distinguishable phases, with LHCSR3 participating in the more rapid (<2 min) phase. This LHCSR3-mediated qT was sensitive to physiological levels of H2O2, which accelerated qE induction, revealing a way that may help C. reinhardtii tolerate a sudden increase in light intensity. Overall, a clear mechanistic overlap between qE and qT is shown.
Achieving photosynthetic efficiency under naturally fluctuating light intensities requires mechanisms that can rapidly switch between highly efficient light absorption and dissipation of excess-absorbed light energy. Otherwise over-excited reaction centres lead to the elevated formation of potentially damaging reactive oxygen species (ROS) and photoinhibition1,2,3. Non-photochemical quenching is a collective name for the mechanisms that regulate energy transfer to the photosystem reaction centres, thereby protecting from photoinhibition.
The most rapidly inducible component of NPQ is qE, which is regulated by the pH of the thylakoid lumen. A low pH leads to protonation of a LHC-type protein triggering the switch between light harvesting and excess light-energy dissipation4,5,6,7,8,9. In higher plants this LHC-type protein is PsbS10, whereas in Chlamydomonas reinhardtii Light-Harvesting-Complex-Stress-Related-3 (LHCSR3) is involved11. Arabidopsis thaliana or C. reinhardtii mutants deficient in PsbS or LHCSR3, respectively, are both referred to as npq4 and have severely diminished qE under excess light10,11. Using npq4 and dissipaters of the trans-thylakoid pH gradient (ΔpH) it has been shown that qE protects from ROS production and photoinhibition under excess light12,13,14. Expression of the gene coding for LHCSR3 (previously referred to as LI818) follows diurnal cycles in day/night grown photoautotrophic cells15, and can be rapidly up-regulated when cells are subjected to high light under ambient CO211, conditions that lead to excess light absorption and a need for qE.
State transitions (qT) are the reversible associations of LHCs, mainly LHCII, with PSII and PSI. Transitioning from state I, where LHCII is coupled to PSII, to state II is regulated by a thylakoid-bound kinase, which phosphorylates LHC proteins when the plastoquinone (PQ) pool becomes reduced. It is an NPQ mechanism that is much more active in algae than higher plants16. In C. reinhardtii up to 80% of LHCII de-couples energy transfer from PSII in state II, with PSI able to use 20% of this light energy17,18. STN7, the higher plant analogue of Stt7 in C. reinhardtii19, has H2O2-sensitive thiol groups20 that are likely conserved in Stt7, as Stt7-mediated LHC phosophoryations are decreased by H2O221. Hydrogen peroxide is a ROS produced in the chloroplast under high light22. Reduction of cysteines 68 and 73 of Stt7 are required for Stt7 kinase activity23, explaining why H2O2 that promotes disulphide bridge formation inhibits LHC phosophorylation21. The Stt7-deficient C. reinhardtii (stt7) is not particularly prone to photoinhibition24, but the npq4stt7–9 double mutant is more light-sensitive than npq413. Recently, it was shown that stt7–9 is a “leaky” mutant and still possesses residual Stt7 kinase activity25, including phosophorylation of LHCSR3, although with severely diminished levels of LHCII phosphorylation13,21.
Together, qT and qE act synergistically for the benefit of photosynthetic efficiency26. While it is clear that qE and qT each have unique mechanistic aspects, a significant component of each requires reorganisation of LHCII and could possess more structural overlap than previously recognised. After all, it has been noticed that LHCSR3 associates to PSI as part of the mobile LHC fraction during qT13,25. Here, using npq4, stt7–9 and stt7-7 (a non-leaky Stt7-kinase deficient mutant) it is shown that LHCSR3 is involved in a de-coupling and re-coupling of energy transfer to PSII during qT. This process was sensitive to H2O2 and, in agreement with an involvement of LHCSR3, the qT of npq4 was much less H2O2-sensitive than wild-type cells. During high light the H2O2-sensitivity of transitioning to state I accelerated qE induction, enabling cells to adjust to high light quicker.
Results
Between the light intensities of 250–2000 μmol quanta m−2 s−1 the qE of npq4 was six times lower than in wild-type cells, a deficiency due to the absence of the light-inducible LHCSR3 protein (Fig. 1). To compare the qT of npq4 and wild-type cells chlorophyll fluorescence at a temperature of 77 K was measured after various light treatments. Chlorophyll excitation at 77 K leads to emission peaks centred at 685 and 715 nm, corresponding to PSII and PSI, respectively, thereby indicating the location of the mobile fraction of LHCII. Dark adapting pre high light-treated wild-type cells led to a decrease of fluorescence at 685 nm, typical of a transition to state II and a de-coupling of energy transfer away from PSII, which also occurred in npq4 (Fig. 2a). Continued respiration and ATP consumption in the dark leading to an imbalanced ATP:NADPH ratio induces NADPH reduction of the PQ pool (chloro-respiration) that activates Stt7 kinase and induction of state II19,27. Re-exposing these cells to light for 3 min, thereby re-oxidising the PQ pool, increased fluorescence at 685 nm relative to 715 nm (Fig. 2a), as expected in state I when LHCII couples energy transfer to PSII. Transitioning from state I (under high light) to state II (due to dark) and back to state I (due to light) was accompanied by a major increase then progressive decreases in LHC phosphorylation of wild-type cells (Fig. 2b), as expected19. Only PSBC (CP43) and PSBD (D2) proteins of the PSII complex were detectably phosphorylated by stt7–9 in the dark (Supplementary Figure S1), as expected25.
Figure 1. The qE phenotype of npq4.
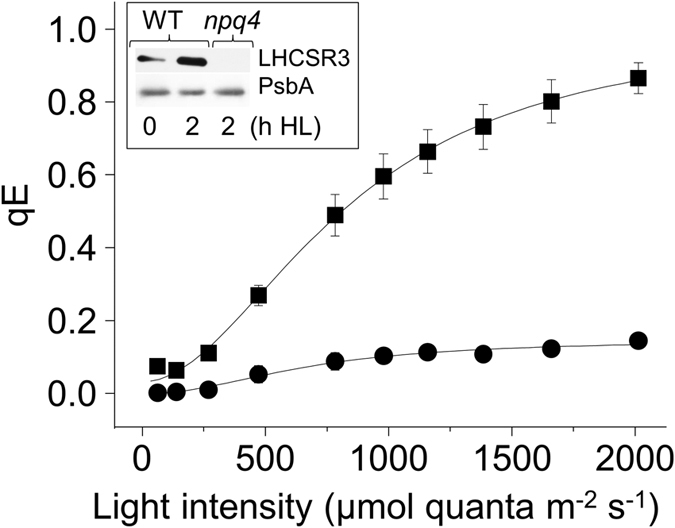
The NPQ parameter of qE was measured at the indicated light intensities in wild-type (squares) and npq4 (circles). Cells were pre-treated with high light for 2 h and recovered for 1.5 h before measurements, n = 3 ± SD. The western blot inset shows the accumulation of LHCSR3 in 0 and 2 h treated wild-type cells and its absence in npq4. Supplementary Figure S5 shows an uncropped blot. The D1 subunit of PSII (PsbA) is shown as loading control.
Figure 2. Characterisation of qT in wild-type and npq4.
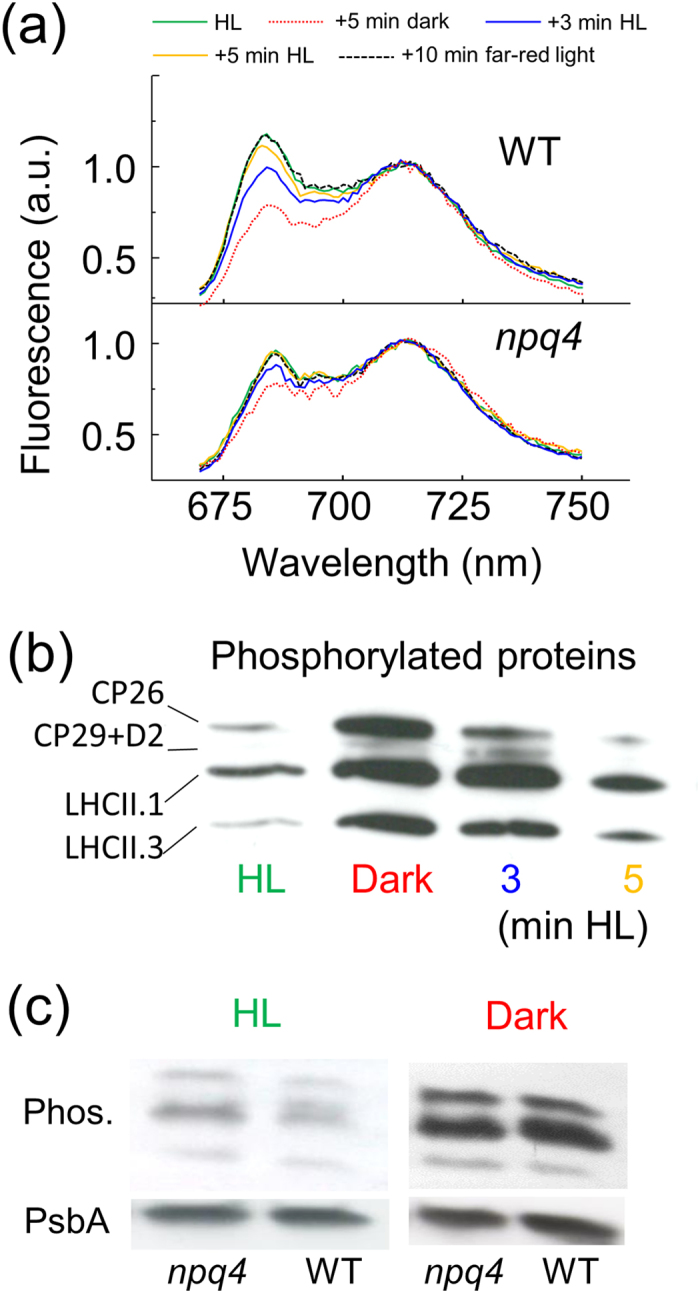
(a) Low-temperature (77 K) chlorophyll fluorescence emission spectra (ex: 440 nm) of wild-type in state I after 2 h high light pre-treatment (HL; green), in state II after a subsequent 5 min dark (red dotted) and then re-transitioning to state I by exposure to 3 min (blue) and 5 min (yellow) high light, and a further 10 min far-red light (black dashed). The changes in npq4 are shown below wild-type and all spectra were normalised at 715 nm. (b) Modification in the level of phosphorylation of thylakoid proteins in wild-type during transitioning between states I and II. Cells were treated as in (a) and phosphorylated proteins were detected by immunoblotting with phospho-threonine antibody. (c) Levels of thylakoid protein phosphorylation (Phos.) of wild-type and npq4 in high light (HL) and after a subsequent 4 min of darkness (Dark). Supplementary Figure S6 shows an uncropped blot. The D1 subunit of PSII (PsbA) blotted from the same membrane is shown as a loading control.
In a comparison of npq4 and wild-type cells in state I, the 77 K chlorophyll fluorescence at 685 nm (i.e. LHCII coupling with PSII) was noticeably less in npq4 (Fig. 2a). A decrease in fluorescence from PSII could be due a higher Stt7 activity preventing cells from attaining state I. However, no major differences in LHC phosphorylation were observed between npq4 and wild-type cells in state I or state II (Fig. 2c). Other explanations for a decreased fluorescence from PSII could be photoinhibition, smaller antenna size and higher qE, but the similar maximum quantum yield of PSII (Fv/Fm) after pre-high light treatment indicated limited differences in photoinhibition (Table 1), the insignificantly different maximum chlorophyll fluorescence (Fm) measured in the presence of DCMU showed equal antenna size (Table 1) and qE is not higher in npq4 (Fig. 1). Therefore, a lack of fully achieving state 1 in npq4 could be explained by an absence of LHCSR3 restricting energy coupling to PSII. It is important to note that while traditionally a transition to state II assumed that most LHCII migrated to PSI this is now considered unlikely18. The definition of state II used here is that LHCII has disassociated energy transfer from PSII regardless of any association to PSI.
Table 1. The differences in pigment composition and chlorophyll fluorescence parameters of wild-type and npq4 cells after the high light pre-treatment.
| WT | SD | npq4 | SD | % from WT | |
|---|---|---|---|---|---|
| Lutein | 15.06 | 1.32 | 16.03 | 0.89 | +6.4 |
| Total VAZ | 9.09 | 0.43 | 10.31 | 0.77 | +13.4* |
| Chl. a:b | 2.49 | 0.11 | 2.70 | 0.08 | +8.5* |
| Fv/Fm | 0.63 | 0.01 | 0.61 | 0.01 | −3.8* |
| Fo (DCMU) | 0.100 | 0.004 | 0.107 | 0.003 | +7.0 |
‘WT’ = wild-type, ‘Total VAZ’ = total xanthophyll pool of violaxanthin, antheraxanthin and zeaxanthin calculated on a mol basis and expressed as molx100:mol total chlorophyll, as for lutein. ‘Chl. a:b’ = ratio of chlorophyll a:b calculated on a mol basis, ‘Fv/Fm’ = maximum quantum yield of PSII, ‘Fo (DCMU)’ = Fo measured in cultures at 10 μg mL−1 chlorophyll in the presence of 10 μM DCMU, ‘% from WT’ was calculated as (npq4-WT)/WT × 100 and ‘*’ corresponds to a significant difference (P < 0.05), n = 4 ± SD.
At room temperature chlorophyll fluorescence is primarily from PSII. By following changes Fm, qT can be measured in vivo by increases and decreases in Fm that correspond to transitioning to state I and state II, respectively. Cells were placed in state I with far-red light until Fm no longer increased. After switching off the far-red light and placing cells in darkness, Fm decreased as cells transitioned to state II. The greatest decrease in Fm occurred during the first minute and in npq4 this decrease was 59% less than in wild-type (Fig. 3). Therefore, the absence of LHCSR3 affected measurements of qT in mechanism completely separate to qE (i.e. in darkness). It was observed that npq4 has a slightly higher chlorophyll a:b ratio and a larger size of the xanthophyll cycle pool than wild-type cells (Table 1), indicating there are some minor differences in the LHC composition of npq4, as noted previously11, but the PSII antenna size was equal (Table 1). In stt7–9 there was also a small, but significant decrease in Fm within the first 0.5 min after switching off the far-red light, while in stt7-7 this decrease was even smaller. The linear decrease in Fm during 2–4 min (inset Fig. 3) was insignificantly different between npq4 and wild-type, and can be attributed to Stt7 kinase-mediated qT by its near absence in stt7–9 and stt7-7. In the presence of 0.1 mM H2O2 the initial (0–1 min) rate of Fm decrease was significantly lowered by 20% in wild-type cells, but insignificantly lowered in npq4 (Fig. 3). H2O2 had less effect on the slower decrease in Fm (2–4 min) of wild-type and npq4 cells.
Figure 3. The state I to II transition of qT separated into the LHCSR3-mediated and Stt7 kinase-mediated components and effects of H2O2.
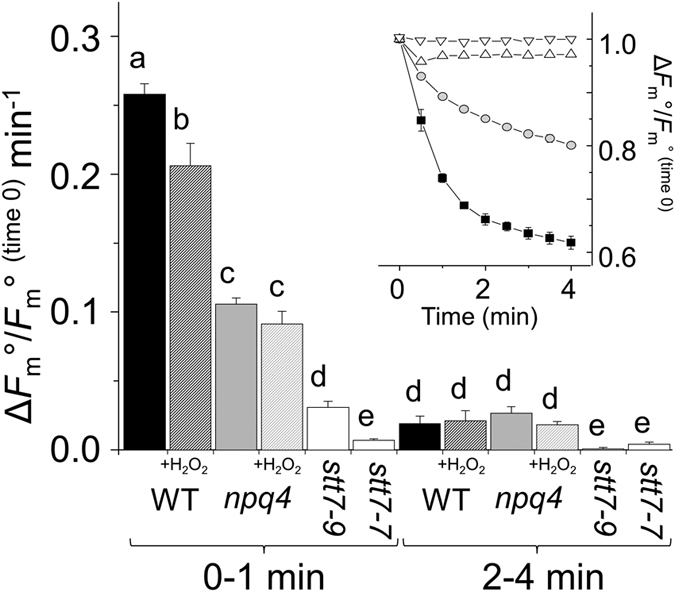
High light-treated cells were subjected to far-red light to fully induce state I (Fm°FR). State II conditions were activated by placing cells in darkness and the decrease in Fm° was followed (see inset) in wild-type (WT; squares), npq4 (circles), stt7-7 (downward triangles) or stt7–9 (upward triangles). The bar chart shows decrease rates in Fm° of wild-type (black), npq4 (grey) or stt7-7/stt7–9 (white) during 0–1 min or 2–4 min in the absence (solid) or presence (white diagonal-stripe) of 0.1 mM H2O2 added 1 min before measurements. Data was normalised to Fm°FR at 0 min. Different letters indicate significant differences (P < 0.05), n = 4 ± SD.
Deciphering chlorophyll fluorescence for measuring qE in C. reinhardtii can be problematic because of the large overlap of qT. For example, qE apparently decreased to negative values during a quenching analysis because Fm’ (Fm measured in the light) increased above Fm° (Fm measured in the dark) (Fig. 4). This can be explained by cells transitioning from state II to state I when they are transferred from dark to light, as shown by 77 K chlorophyll fluorescence in Fig. 2a. In low light-acclimated wild-type cells, which only accumulated low levels of LHCSR3 (Fig. 1), there was an absence of the rapid light-induced rise in Fm’ (Supplementary Figure S2). Simultaneously measuring net O2 production during the quenching analysis helped separate the qE and qT responses. High light treated cells were dark-adapted to allow qE to relax and to place them in state II. Subsequently, during the quenching analysis, despite differences in the time before qE was induced (Fig. 4a) it only occurred when net O2 production ceased (Fig. 4b). Most likely, qE became induced once CO2 became depleted21. Longer recovery times permitted more CO2 to dissolve into the media that became CO2-depleted by photosynthesis during the high light pre-treatment. Dark-adapting wild-type cells for only 3 min led to qE being induced before cells fully transitioned to state I, whereas 20–45 min dark adaption enabled sufficient recovery time to reveal two qT kinetics: First, an initial rapid increase in Fm’ between 0.5–2 min of illumination that partially occurred in stt7–9 and to a lesser extent in stt7-7, but was absent in npq4, and a second slower increase that was absent in stt7–9 and stt7-7, but present in npq4 (Figs 4c and 5). To summarise, with the help of the arrows in the quenching analysis shown in Figs 4c and 5, the initial NPQ phases of wild-type cells can be assigned in order of their occurrence to an immediate LHCSR3-dependent qE followed by an LHCSR3-dependent rapid qT transition to state I that overlaps with the slower Stt7-mediated qT transition to state I.
Figure 4. The state II to I transition of qT and qE induction during dark to light exposure.
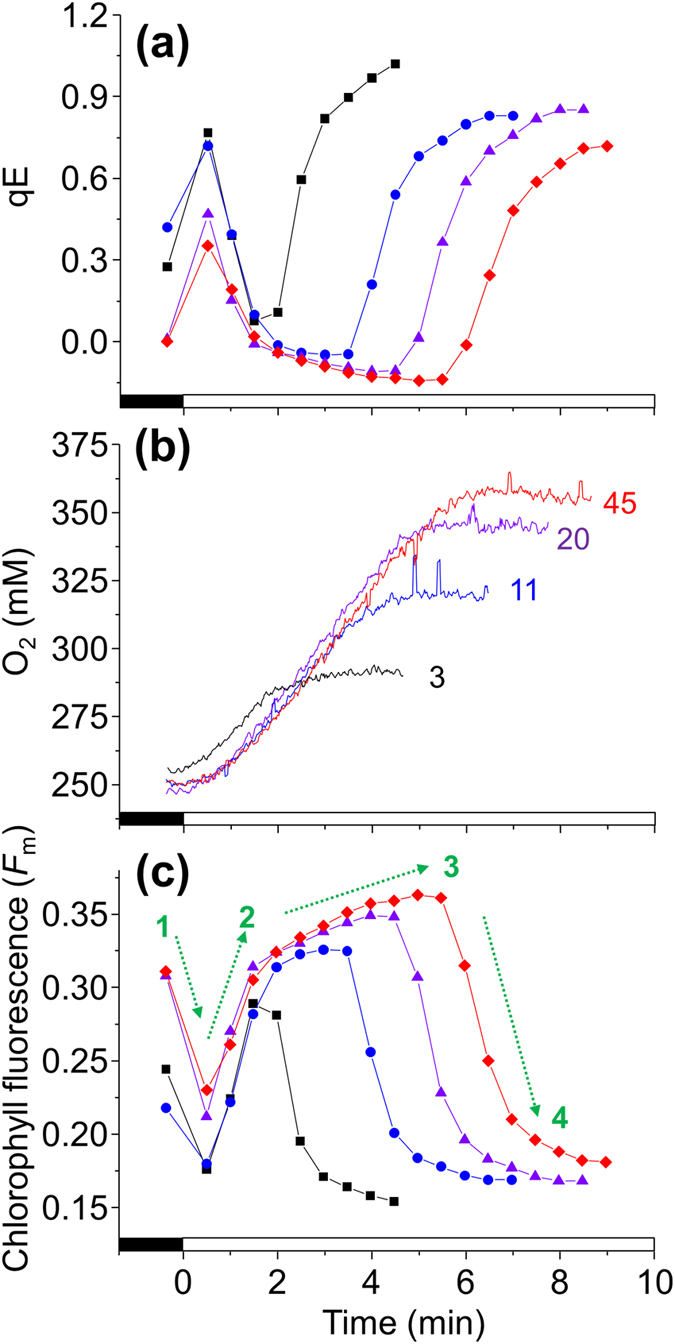
Pre high light-treated wild-type cells were placed in state II by dark-adapting for various intervals. Actinic light at 474 μmol quanta m−2 s−1, as used to induce a transition to state I and activate qE, is indicated by the white bar on the X-axis. (a) The qE of cells dark adapted for 3 min (squares), 11 min (circles), 20 min (triangles) or 45 min (diamonds), calculated for all using the Fmo after 45 min dark adaptation. (b) O2 content of the media (lines are labelled with the time of dark adaption) simultaneously measured during a fluorescence quenching analysis. (c) The Fm’ values used to calculate qE using the same symbols in (a). In (c) the dashed green arrows of Fm changes indicate 1) rapid qE of state II cells, 2) rapid qT transition to state I, 3) slower qT transition to state I, and 4) qE of state I cells (see text for details).
Figure 5. The NPQ phases during dark to light exposure separated into the LHCSR3-mediated and Stt7 kinase-mediated components.
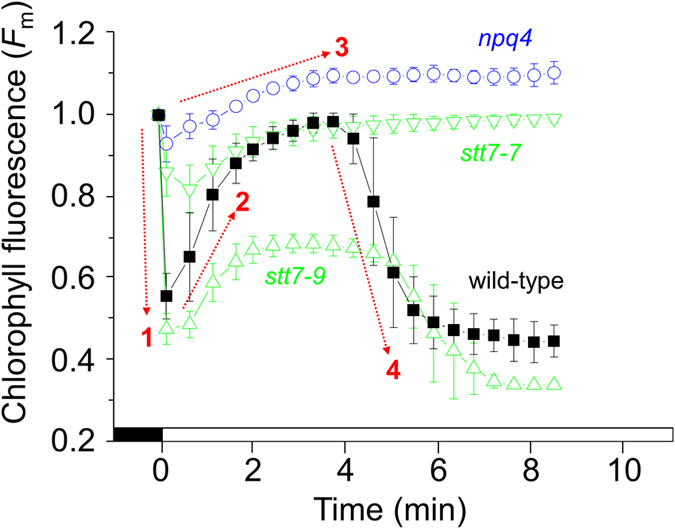
Wild-type (squares), npq4 (circles), stt7–9 (upward triangles) and stt7-7 (downward triangles) were pre high light-treated and then dark-adapted for 15 min inducing state II conditions. Subsequently, cells were treated with 474 μmol quanta m−2 s−1, as indicated by the white bar on the X-axis, to induce state I before qE became induced. Data are normalised to Fm° at 0 min. The dashed red arrows of Fm changes in wild-type cells indicate 1) rapid LHCSR3-dependent qE, 2) rapid LHCSR3-involved qT transition to state I, 3) slower Stt7-mediated qT transition to state I, and 4) LHCSR3- and Stt7-dependent qE of state I cells (see text for details).
With further light treatment qE became induced, as shown by the decrease in Fm’ that could be prevented by the ΔpH-dissipater nigericin (Supplementary Figure S3). Despite that stt7-7 had no less LHCSR3 than stt7–9 (Supplementary Figure S4), Fm’ of stt7-7 did not decrease by the time qE had been induced in wild-type or stt7–9 (Fig. 5). Therefore, although qE was LHCSR3 dependent (i.e. absent in npq4) there was further control mediated by Stt7 only apparent in the non-leaky stt7-7. When the quenching analysis of wild-type cells was made in the presence of catalase the light-induced transition to state I was delayed21, as was the induction of qE (Fig. 6). The same slowing of qE induction from the addition of catalase was also observed with stt7–9, but not stt7-7 that was unable to induce qE during the analysis (Supplementary Figure S4).
Figure 6. H2O2 accelerates the induction of qE.
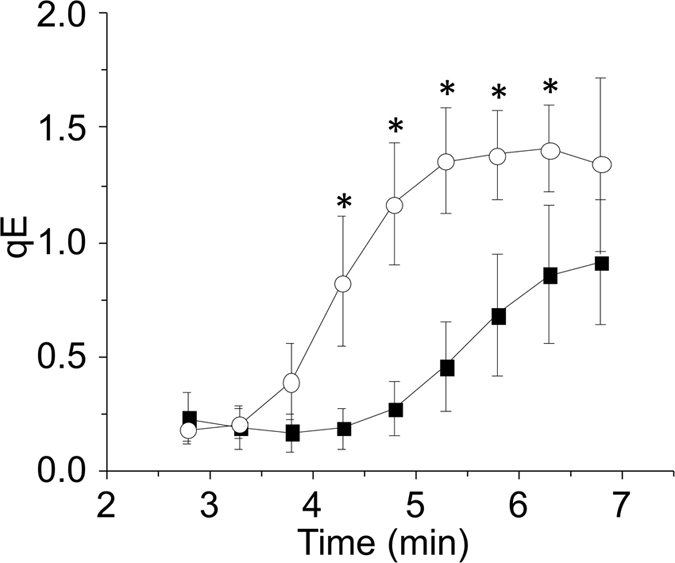
Wild-type cells were pre high light-treated and then dark-adapted for 15 min in the presence (closed squares) or absence (open circles) of 500 U mL−1 catalase. Cells were subsequently treated with 474 μmol quanta m−2 s−1 to induce qE. Significant differences (P < 0.05) in the presence or absence of catalase at each interval are indicated by *.
Discussion
The NPQ mechanisms of qE and qT have each been characterised under distinct conditions, leading to the notion of completely unique processes. For example, qT has been investigated in conditions such as anoxia in the dark17,18,19,27, which is far away from the excess light required for inducing qE. However, Tokutsu and Minagawa5 showed that the majority of LHCSR3 in high light-treated C. reinhardtii was associated with detached LHCII, a situation that could have derived from either qE or qT. Moreover, others have shown LHCSR3 attached to the PSI-supercomplex13,25, fitting with a role for LHCSR3 in qT. As discussed below, our data would fully support a role for LHCSR3 in energy coupling of LHCII to PSII as part of qT.
Placing C. reinhardtii from high-light or far-red light into darkness activates chlororespiration, a reduction of the PQ pool and transition to state II13,27. Two kinetically separate phases were evident in wild-type cells during this transition, with npq4 retarded in the initial rapid decrease of Fm° (Fig. 3). However, the later linear and slower decrease of Fm° from 2–4 min, absent in stt7 mutants, was equally present in npq4 and wild-type alongside equally phosphorylated LHCs after 4 min dark in npq4 and wild-type (Fig. 2c). In summary, npq4 was inhibited in the rapid de-coupling of energy to PSII during a transition to state II, revealing the involvement of LHCSR3, but npq4 was not affected in the slower de-coupling of energy that was attributable to Stt7 kinase.
A role for LHCSR3 in energy coupling to PSII during transition to state I was also explored. Exposing dark-treated cells in state II to actinic light induces transition to state I13, as shown by changes in chlorophyll fluorescence at 77 K (Fig. 2a). A light-induced transition to state I can be called the “S” (semi-steady state) to “M” (maximum) rise when using the so-called O-J-I-P-S-M nomenclature28. Here, it was observed during a quenching analysis that the increase in Fm’ of wild-type cells possessed two kinetically separate phases; a rapid initial increase partially present in stt7 mutants, but totally absent in npq4 (Fig. 5), and a second slower increase over several minutes (Fig. 4c) that occurred alongside a decrease in LHC phosphorylation (Fig. 2b), explaining its absence in stt7 mutants (Fig. 5). A lack in the rapid Fm’ increase early in the quenching analysis by npq4 confirms an involvement of LHCSR3, and also explains why a high light pre-treatment to induce LHCSR3 was required to see this phenomenon (Supplementary Figure S2). This also explains why the rapid increase in Fm’ was also observed in stt7–9, and to a lesser extent in stt7–7 (Fig. 5). It is known that stt7–9 is a leaky mutant with residual Stt7 activity25. However, measuring qT by shifting far-red light-treated cells to dark showed stt7-7 and stt7–9 behaved very similar (Fig. 3). After all, despite its leaky nature, stt7–9 cannot phosphorylate the LHCII protein LHCBM5 in state II conditions25 and cannot perform Stt7-mediated qT13,29. The differences in the behaviour of stt7-7 and stt7–9 during the quenching analysis are therefore only apparent under actinic light. Stromal residues of the LHCSR3 N-terminal (Ser-26, Ser-28, Thr-32, Thr-33, and Thr-39), can be phosphorylated in wild-type and stt7–9, but not in a non-leaky Stt7-deficient mutant25. We suggest that Stt7 phosphorylation of LHCSR3 is involved in the LHCSR3-mediated and light-dependent qT, which could explain the smaller increase in Fm’ early during the quenching analysis by stt7-7 compared to stt7–9. It is tempting to speculate that such LHCSR3 phosphorylations may also explain the deficiency of qE in stt7-7. However, a difference in the phosophorylation level of LHCB4, or other proteins that occur in stt7–9, compared with a non-leaky Stt7 mutant25, may also be responsible.
In summary, the rapid and slower decreases of Fm° during a transition to state II (Fig. 3 inset) kinetically mirrored the rapid and slower increases in Fm’ during transition to state I (Fig. 4c). With the use of npq4, stt7-7 and stt7–9 we are able to deduce that LHCSR3 is required for the more rapid transitions of qT, while only Stt7 kinase activity was involved in the slower transitions. Furthermore, Stt7-mediated phosphorylations are also involved in qE.
Previously, it has been shown that STN7 kinase of Arabidopsis has H2O2-sensitive exposed thiol groups20 and that LHC phosphorylations mediated by Stt7 kinase in C. reinhardtii were inhibited by H2O221. It is now apparent that the more rapid transition to state II, which involves LHCSR3, is more sensitive to H2O2 than the slower transition to state II, which involves only Stt7 kinase (Fig. 3). Furthermore, the involvement of LHCSR3 explains why measurements of npq4 were much less influenced by H2O2 (Fig. 3). An explanation to why LHCSR3-mediated qT is particularly sensitive to H2O2 could be that a smaller change in phosphorylation levels leads to a larger level of regulation than LHCII phosphorylation, which merits further investigation. A transition in the reverse direction was also sensitive to H2O2, as shown by the delayed transition to state I in wild-type cells treated with catalase. This phenomenon can be explained by H2O2 slowing Stt7 kinase activity, which accelerates the transition to state I21. As this effect was seen after removing H2O2 rather than by its addition, this level of regulation is clearly operational under standard lab conditions and with physiological levels of H2O2. Transitioning to state I during a sudden increase in light intensity has been previously described as a mechanism that facilitates qE induction by increasing light absorption by PSII13. Here we showed that catalase delayed the onset of qE by approximately 2.5 min in wild-type (Fig. 6) and stt7–9 (Supplementary Fig. 4), both of which can phosphorylate LHCSR3. In conclusion, H2O2 production in the chloroplast can benefit C. reinhardtii by adjusting to a rapid increase in light intensity through a process involving Stt7 and LHCSR3, and potentially Stt7-mediated phosphorylation of LHCSR3.
Materials and Methods
Strains and Growth Conditions and high light pre-treatments
Chlamydomonas reinhardii wild type (wild-type) strain T222 (in the 137C background), stt7–9 (a leaky mutant with an estimated 6-fold decrease in Stt7 kinase activity25) and stt7-7 (a totally Stt7-deficient mutant, J-D. Rochaix, personal communication) were gifts from J-D. Rochaix, University of Geneva. npq411 (CC-4614) was purchased from the Chlamydomonas Centre (www.chlamycollection.org). Cultures were initiated in Tris-Acetate-Phosphate media (TAP)30, adjusted to pH 7.0, and grown mixotrophically under low light (50 μmol quanta m−2s−1). To transfer cells to photoautotrophic conditions TAP cultures were pelleted for 2 min at 1600 g and suspended in Tris-HCl-Phosphate media (THP; identical to TAP except the pH was adjusted to 7.0 with HCl rather than acetic acid) and cultivated under low light while being bubbled with sterile air, achieved with a 0.22 μM air-filter. Cells were in THP for at least 24 h before experiments began, which is well beyond the time for residual acetate to be consumed that can affect photosynthetic performance and ROS production31. Liquid cultures were rotated at 80 rpm at 20 °C and kept in the exponential growth phase below 5 × 106 cells ml−1 by regular dilution. Adjustment to 10 μg chlorophyll ml−1 was made immediately before pre-high light treatments.
High light was provided by a 250 W compact fluorescent lamp and cultures were kept between 20–25 °C with fan-assisted cooling. The high light intensity measured at the top and bottom of the culture was 300 and 200 μmol quanta m−2 s−1, respectively, and unless stated otherwise cultures were pre-high light-treated for 2 h.
Photosynthetic pigments
Carotenoids were measured from 10 mg of lyophilized cells extracted in 1 mL of ice-cold acetone by shaking (TissueLyser II, Qiagen, Düsseldorf, Germany) at 30 Hz for 2 min with two 2 mm glass beads before centrifugation at 26,000 g for 45 min. Ten μl of the supernatant was injected using an Agilent 1100 HPLC system equipped with a LiChrospher 100 RP-18 (5 μm) column (Agilent Technologies, Santa Clara, California, USA). Peak identity and quantification was made against individual standards with absorbance at 440 nm. Total chlorophyll and chlorophyll a and b were measured according to32 in 80% acetone.
Photosynthetic Measurements
Pulse amplitude-modulated (PAM) chlorophyll fluorescence measurements were made with a PAM 2500 (Walz GmbH, Effeltrich, Germany). Maximum chlorophyll fluorescence (Fm) was measured with a 200 ms saturating pulse. Cultures of 1.5 mL were constantly stirred with a magnetic bar during measurements. For measuring the light dependency of qE induction cells were first allowed to recover from high light for at least 1 h and then treated with far-red light to fully achieve maximum Fm (Fmo) Increasing intensities of light were provided for 1 min intervals after which Fm’ (Fm under actinic light) was measured in order to calculate qE via (Fmo − Fm’)/Fm’. For measuring the state II to I transition, cells were treated with far-red light to achieve state I, as observed when Fmo no longer increased (typically 10 min when saturating pulses were kept 90 s apart). Induction of state II was measured by following the decrease in Fm after the far-red light was switched off. H2O2 was added 1 minute before measurements from a stock of 100 mM. For splitting the qE and qT responses cells were dark-adapted for various times, as stated in the Figure legend, before treating at a light intensity of 474 μmol quanta m−2 s−1. The Fm’ was measured every 30 s. To observe the effects of nigericin the cell wall-less wild-type strain (cw15) was used, because nigericin can enter its cells and the strain possesses typical LHC phosphorylation patterns and can accumulate LHCSR3 (Roach, unpublished). Nigericin was used at 10 μM from a 10 mM stock dissolved in methanol. To assess PSII antenna size via Fmo cells were first treated for 1 min in the dark with 10 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) from a 10 mM stock dissolved in methanol.
Chlorophyll fluorescence emission between 650–750 nm was measured at 77 K with a Cary Eclipse fluorescence spectrometer (Varian, Mulgrave, Australia) at an excitation of 440 nm (5 nm slit width) in samples capillary-loaded in glass Pasteur pipettes and immediately frozen in N2(l) before measurement.
Analysis of LHCSR3, PsbA and threo-phosphorylated protein levels
Total cellular proteins were extracted in 2% SDS in 100 mM TRIS-HCl, pH 6.8, containing a protease inhibitor cocktail (Complete Mini, Roche Diagnostics, Switzerland). Proteins were quantified using the bicinchoninic acid assay (Sigma-Aldrich, St Louis MO, USA), loaded at 20 μg protein/sample and separated by PAGE using 12% acrylamide gels at 40 mA for 1.5 h. For western blotting separated proteins were transferred to nitrocellulose membranes at 40 mA/gel for 1 h, which were subsequently blocked in 5% fat-free milk powder before incubating with the LHCSR3 (Agrisera, Sweden) or anti-phospho-threonine antibody (Cell Signalling Technologies, USA) at 1:10,000 dilution or PsbA antibody (Agrisera, Sweden) at 1:25,000 dilution. The peroxidase-coupled antibodies were visualised with enhanced chemiluminescence (Amersham, GE Healthcare, UK) and light sensitive film (Amersham, GE Healthcare, UK).
Statistics
Significant differences at P < 0.05 were calculated using IBM SPSS (v.21) and one-way ANOVA with Tukey’s post-hoc test.
Additional Information
How to cite this article: Roach, T. and Na, C. S. LHCSR3 affects de-coupling and re-coupling of LHCII to PSII during state transitions in Chlamydomonas reinhardtii. Sci. Rep. 7, 43145; doi: 10.1038/srep43145 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by the National Research Foundation of Korea grant NRF-2013R1A/1A2010178 and the Tiroler Wissenschaftsfonds grant UNI-0404/1571.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.R. conceived and designed the experiments. T.R. and C.S.N. conducted the experimental work and analysed the data. T.R. wrote the paper.
References
- Hideg Ev., Spetea C. & Vass I. Singlet oxygen production in thylakoid membranes during photoinhibition as detected by EPR spectroscopy. Photosynth. Res. 39, 191–199 (1994). [DOI] [PubMed] [Google Scholar]
- Kornyeyev D., Logan B. A. & Holaday A. S. Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct. Plant Biol. 37, 943–951 (2010). [Google Scholar]
- Roach T. & Krieger-liszkay A. Regulation of Photosynthetic Electron Transport and Photoinhibition. Curr. Protein Pept. Sci. 15, 351–362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori N., Roy L. M., Opacic M., Durand G. & Croce R. Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: the C-terminus of LHCSR is the knob of a dimmer switch. J. Am. Chem. Soc. 135, 18339–42 (2013). [DOI] [PubMed] [Google Scholar]
- Tokutsu R. & Minagawa J. Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 110, 10016–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballottari M. et al. Identification of pH-sensing sites in the Light Harvesting Complex Stress-Related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J. Biol. Chem.doi: 10.107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Wentworth M. & Ruban A. Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett. 579, 4201–6 (2005). [DOI] [PubMed] [Google Scholar]
- Betterle N. et al. Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–66 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R., Miloslavina Y., Nilkens M. & Jahns P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 (2009). [Google Scholar]
- Li X. P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000). [DOI] [PubMed] [Google Scholar]
- Peers G. et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–521 (2009). [DOI] [PubMed] [Google Scholar]
- Roach T. & Krieger-Liszkay A. The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochim. Biophys. Acta 1817, 2158–2165 (2012). [DOI] [PubMed] [Google Scholar]
- Allorent G. et al. A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25, 545–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T., Miller R., Aigner S. & Kranner I. Diurnal changes in the xanthophyll cycle pigments of freshwater algae correlate with the environmental hydrogen peroxide concentration rather than non-photochemical quenching. Ann. Bot. 116, 519–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard F., Richard C. & Guertin M. The Chlamydomonas reinhardtii LI818 gene represents a distant relative of the cabI/II genes that is regulated during the cell cycle and in response to illumination. Plant Mol. Biol. 32, 461–73 (1996). [DOI] [PubMed] [Google Scholar]
- Lemeille S. & Rochaix J.-D. State transitions at the crossroad of thylakoid signalling pathways. Photosynth. Res. 106, 33–46 (2010) [DOI] [PubMed] [Google Scholar]
- Nagy G. et al. Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc. Natl. Acad. Sci. USA 111, 5042–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünlü C. et al. State transitions in Chlamydomonas reinhardtii strongly modulate the functional size of photosystem II but not of photosystem I. Proc. Natl. Acad. Sci. USA 111, 3460–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depège N., Bellafiore S. & Rochaix J.-D. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575 (2003). [DOI] [PubMed] [Google Scholar]
- Muthuramalingam M., Matros A., Scheibe R., Mock H.-P. & Dietz K.-J. The hydrogen peroxide-sensitive proteome of the chloroplast in vitro and in vivo. Front. Plant Sci. 4, 54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T., Na C. S. & Liszkay A. High light-induced hydrogen peroxide production in Chlamydomonas reinhardtii is increased by high CO2 availability. Plant J. 81, 759–766 (2015). [DOI] [PubMed] [Google Scholar]
- Asada K. Production and Scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K. et al. Trans-Membrane Signaling in Photosynthetic State Transitions: Redox- and Structure- Dependent Interaction In Vitro between Stt7 Kinase and the Cytochrome b6f Complex. J. Biol. Chem.jbc.M116.732545, doi: 10.1074/jbc.M116.732545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann M. M. et al. Isolation and characterization of photoautotrophic mutants of Chlamydomonas reinhardtii deficient in state transition. J. Biol. Chem. 274, 30987–94 (1999). [DOI] [PubMed] [Google Scholar]
- Bergner S. V. et al. State transition7-dependent phosphorylation is modulated by changing environmental conditions and its absence triggers remodeling of photosynthetic protein complexes. Plant Physiol. 168, 615–634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M. & Aro E.-M. Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci. 16, 126–31 (2011). [DOI] [PubMed] [Google Scholar]
- Bulte L., Gans P., Rebeille F. & Wollman F. ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim. Biophys. Acta - Bioenerg. 1020, 72–80 (1990). [Google Scholar]
- Kodru S. et al. The slow S to M rise of chlorophyll a fluorescence reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth. Res. 125, 219–231 (2015). [DOI] [PubMed] [Google Scholar]
- Cardol P. et al. Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc. Natl. Acad. Sci. USA 106, 15979–15984 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H. The Chlamydomonas SourcebookVol. 1 (eds Harris E. H., Stern D. B. & Witman G.) Ch. 8, 241–292 (Academic Press, 1989). [Google Scholar]
- Roach T., Sedoud A. & Krieger-Liszkay A. Acetate in mixotrophic growth medium affects photosystem II in Chlamydomonas reinhardtii and protects against photoinhibition. Biochim. Biophys. Acta 1827, 1183–1190 (2013). [DOI] [PubMed] [Google Scholar]
- Porra R. J., Thompson W. A. & Kriedemann P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta - Bioenerg. 975, 384–394 (1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


