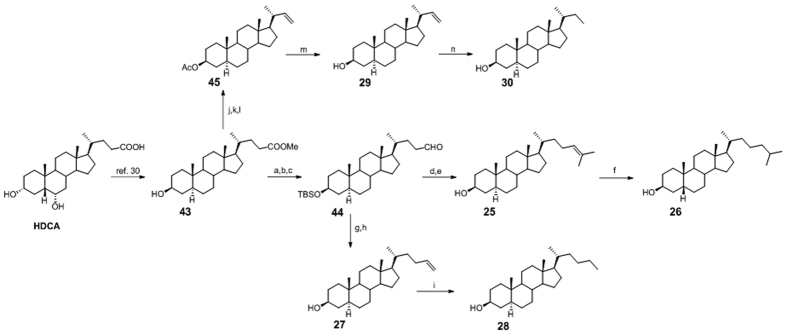
Figure 11. Preparation of Subset B derivatives. Linear C23/C25 and branched C26 aliphatic side chains on 3β-hydroxy-6-deoxy-5α-hyodeoxycholane scaffold.
Reagents and conditions: (a) 2,6-lutidine, t-butyldimethylsilyltrifluoromethanesulfonate, CH2Cl2, 0 °C; (b) LiBH4, MeOH dry, THF, 0 °C; (c) DMSO, oxalyl chloride, TEA dry, CH2Cl2, −78 °C, 34% over three steps; (d) n-BuLi, isopropyl triphenylphosponium iodide, THF dry, r.t.; (e) HCl 37%, MeOH, 34% over two steps; (f) H2, Pd(OH)2 degussa type, THF:MeOH dry 1:1, quantitative yield; (g) n-BuLi, methyl triphenylphosponium iodide, THF dry, r.t.; (h) HCl 37%, MeOH, 38% over two steps; (i) H2, Pd(OH)2 degussa type, THF:MeOH dry 1:1, quantitative yield; (j) NaOH, MeOH/H2O 1:1 v/v, reflux; (k) Ac2O, pyridine; (l) Cu(OAc)2 H2O, Pb(OAc)4 in toluene dry/pyridine dry, 78%; (m) CH3ONa, CHCl3 dry/MeOH dry 5:3 v/v, 62%; (n) H2, Pd(OH)2 degussa type, THF dry/MeOH dry 1:1 v/v, quantitative yield.