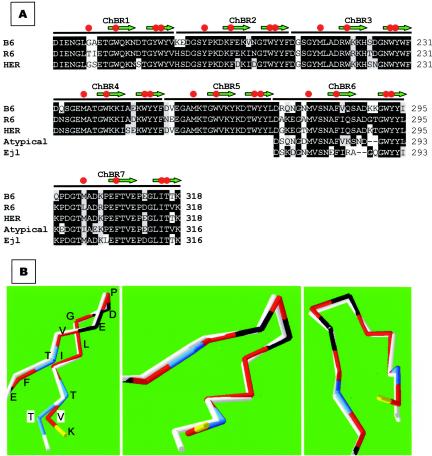
FIG. 6.
Amino acid sequence conservation at the choline-binding domain of typical and atypical amidases. (A) The sequences of the choline-binding domains of LytAB6 (B6) and LytAHER (HER) were compared to that of LytAR6 (R6). The last two choline-binding repeats from Ejl and a consensus sequence from an atypical enzyme (48) were also aligned. Amino acid residues that coincide with those of LytAR6 are shown in black boxes, and conserved substitutions are indicated in a gray background. Red circles and green arrows indicate choline binding residues and the portions of the sequence that form the first and second strands of the hairpins, respectively. (B) Predicted three-dimensional folding of the C-terminal part of LytAB6 (white line). For simplicity, only the α-carbon chains are shown. The Thr317 residue characteristic of LytAB6, LytAHER, and Ejl is highlighted in blue and boxed (T). The corresponding Val residue (V) in LytAR6 is also boxed. The folding of LytAR6 has been experimentally determined (17), and the residues are labeled as follows: acid, black; basic, yellow; polar, blue; nonpolar, red. Three different rotations of the model are shown.