Fig. 2.
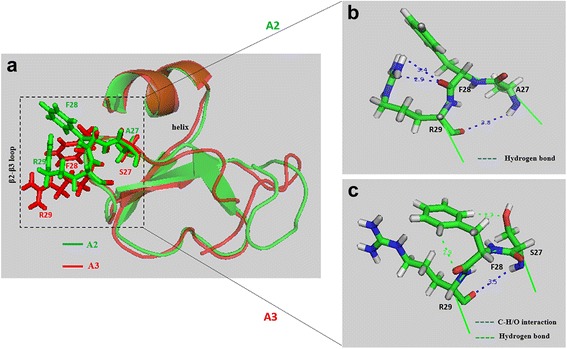
The predicted β2-β3 loop in AvBD-12A2 and AvBD-12A3. a Superimposition of AvBD-12A2 (green) and AvBD-12A3 (red) revealing the structural differences in the β2-β3 loop, a component of CCR2 binding domain. b Enlarged review of the β2-β3 loop in AvBD-12A2. The hydrogen bond between the -C = O group of F28 main chain and the -NH group of R29 side chain causes the arginine residue to fold back. c Enlarged review of the β2-β3 loop in AvBD-12A3. The CHO interactions between S27 -OH groups and the -CH groups on the aromatic ring of F28 result in an outward protrusion of R29 and a parallel twist of F28 aromatic ring. Distance: Å
