Figure 3.
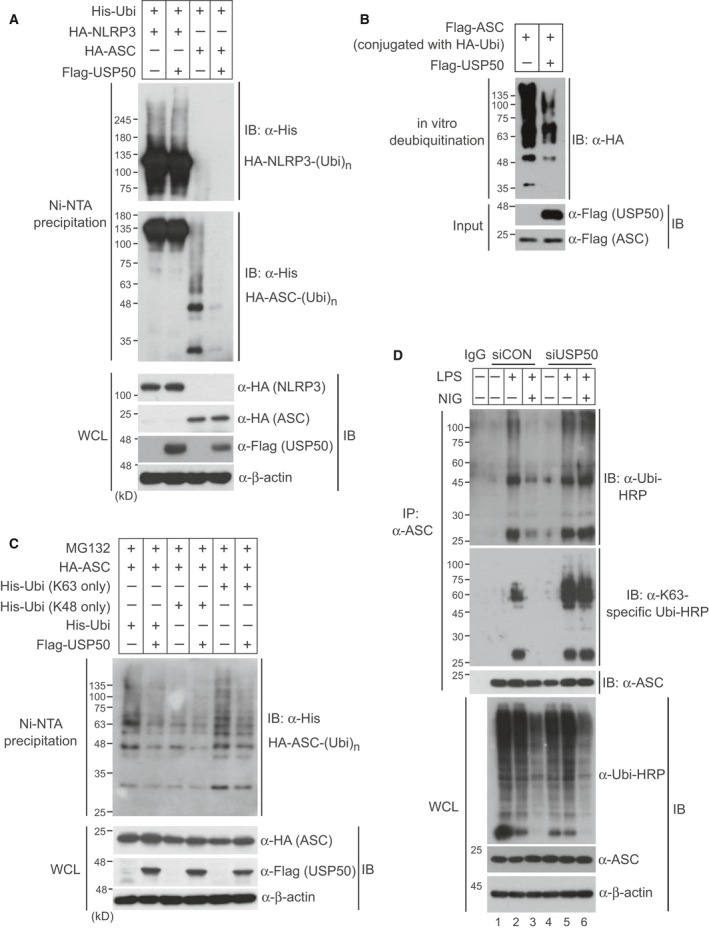
USP50 directly deubiquitinates K63‐linked polyubiquitination of the ASC protein. (A) A plasmid encoding HA‐NLRP3 or HA‐ASC was cotransfected with wild‐type His‐Ubi into HEK293 cells in the absence or presence of Flag‐USP50. After cells were lysed under the denaturing conditions of 6 m guanidine‐HCl, Ni‐nitrilotriacetic acid‐mediated pull‐down assays were performed and the precipitates were subsequently separated with 6% polyacrylamide gel (upper panel) for NLRP3 or 12% polyacrylamide gel (lower panel) for ASC. Separated samples were analyzed by IB with the indicated antibodies. (B) For an in vitro deubiquitination assay, Flag‐ASC protein conjugated with HA‐ubiquitin (HA‐Ubi) was eluted from HEK293 cells which were cotransfected with Flag‐ASC and HA‐Ubi plasmids. Also, Flag‐USP50 protein was eluted from HEK293 cells transfected with the Flag‐USP50 plasmid. The reactions were performed in the indicated combinations and were analyzed by IB with the indicated antibodies. (C) Plasmids encoding His‐Ubi or ubiquitin mutants (K48 only and K63 only) were cotransfected with the HA‐ASC plasmid into HEK293 cells in the absence or presence of Flag‐USP50. After cells were treated with the proteasome inhibitor MG132 for 6 h, Ni‐nitrilotriacetic acid‐mediated pull‐down assays were performed and analyzed by immunoblotting with the indicated antibodies. (D) For in vivo ubiquitination assay of endogenous ASC protein, USP50‐knockdown and control THP‐1 cells were primed with LPS and treated with or without NIG. Cell lysates were immunoprecipitated with anti‐ASC antibody under 1% SDS denaturating condition and subsequently immunoblotted with anti‐ubiquitin‐HRP and anti‐K63 linkage‐specific ubiquitin‐HRP antibodies. WCL were immunoblotted with the indicated antibodies. All data for IB analysis in this figure are representative of at least three independent experiments.
