Abstract
Background
House dust mite (HDM) is the major indoor allergen for allergic diseases such as allergic rhinitis (AR) and asthma. Although sublingual immunotherapy is a curative treatment for HDM‐induced AR, data from large‐scale studies are limited. We evaluated the efficacy and safety of HDM tablets in adolescent and adult patients (aged 12–64 years) with HDM‐induced AR with or without intermittent asthma.
Methods
In a double‐blind trial in Japan, 968 subjects were randomized 1 : 1 : 1 to 300 index of reactivity (IR), 500 IR, or placebo groups. The primary endpoint was the Average Adjusted Symptom Score (AASS) in the last eight weeks of the 52‐week treatment. Secondary endpoints included individual nasal and ocular symptom scores, rescue medication use, and the Japanese Rhinoconjunctivitis Quality of Life Questionnaire (JRQLQ) scores.
Results
The AASS in the last eight weeks of treatment significantly improved in both the 300 IR and the 500 IR groups compared to that in the placebo group (P < 0.001). In the 300 IR group, the onset of action occurred at week 8–10. All four nasal symptoms significantly improved in both active treatment groups; rescue medication use and JRQLQ outcome improved in the 300 IR group. Most adverse events (AEs) were mild, and 16 serious AEs (SAEs) were reported; however, none of them were drug‐related.
Conclusions
One‐year treatment with 300 IR and 500 IR HDM tablets was effective without major safety concerns. The recommended therapeutic dose for AR is 300 IR.
Keywords: allergic rhinitis, Average Adjusted Symptom Score, clinical study, house dust mite, sublingual immunotherapy tablet
Abbreviations
- AASS
Average Adjusted Symptom Score
- AEs
adverse events
- AIT
allergen immunotherapy
- ANCOVA
analysis of covariance
- AR
allergic rhinitis
- ARTSS
average rhinitis total symptom score
- ASS
Adjusted Symptom Score
- FAS
full analysis set
- GCP
good clinical practice
- HDM
house dust mite
- IR
index of reactivity
- JRQLQ
Japanese Rhinoconjunctivitis Quality of Life Questionnaire
- MMRM
mixed‐effects model for repeated measures
- QOL
quality of life
- RTSS
rhinitis total symptom score
- SAEs
serious adverse events
- SCIT
subcutaneous immunotherapy
- SLIT
sublingual immunotherapy
- WAO
World Allergy Organization
House dust mites (HDM), Dermatophagoides pteronyssinus and Dermatophagoides farinae, are common sources of indoor allergens worldwide and can trigger perennial allergic rhinitis (AR) and asthma. The exposure level of HDM allergens is related to the sensitization and manifestation of allergic diseases. Recent research has shown that the body and feces components of HDM exert potent pathogenic activities in two ways: (i) the adaptive immune response, that is, through immunoglobulin E (IgE)‐dependent pathways; and (ii) the innate immune response, that is, through Toll‐like and protease‐activated receptor‐mediated pathways 1. Allergen immunotherapy (AIT) is a causative therapy that can modify the natural course of allergic diseases. Meta‐analysis of clinical studies revealed that HDM subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are effective in AR 2, 3. However, the methodologies employed in the studies were heterogeneous, and sample sizes were limited, which may have complicated our understanding of the clinical significance of HDM AIT 4, 5. We developed a standardized HDM tablet containing extracts of D. pteronyssinus and D. farinae in a 1 : 1 ratio for allergenic activity. In a previous large‐scale adult AR study in Europe, 300 index of reactivity (IR) and 500 IR HDM tablets were found to be effective and safe over a 1‐year treatment 6. A carryover effect was demonstrated as the efficacy of the HDM tablet was maintained after a 1‐year AIT‐free period. The efficacy and safety of another HDM SLIT product in Europe were demonstrated in a recent AR phase 3 study 7. Hence, there is a need to extend the reported clinical benefits of HDM tablets to other parts of the world (Japan, in the present study).
Methods
Clinical trial design
In this randomized, double‐blind, placebo‐controlled, parallel‐group designed study, which included 50 centers in Japan (Japan Pharmaceutical Information Center Clinical Trial Information number: JapicCTI‐121917), patients were randomized 1 : 1 : 1 to a placebo, 300 IR, or 500 IR group (IR, index of reactivity, an in‐house standardization unit). Patients were enrolled in the study between October and December 2012 and were treated for 52 weeks. The study complied with the International Conference on Harmonization Good Clinical Practice (GCP) guidelines and was approved by the Institutional Review Board of each study site.
Participants
Main inclusion criteria were men and women, aged between 12 and 64 years, with a clinical history of HDM‐induced AR for at least 2 years, a positive HDM‐specific serum IgE (≥0.70 Ua/ml), a positive result in a nasal provocation test using a house dust disk, and an Average Rhinitis Total Symptom Score (ARTSS) ≥6 of 15 for 7 days before randomization.
Patients were excluded from the study if they were suspected of symptomatic AR due to allergens other than HDM or high serum‐specific IgE against allergens other than HDM, because high specific IgE titer is indicative of present or new manifestation of symptomatic AR 8. Patients were also excluded if they had mild‐persistent or more severe asthma or required treatment with inhaled corticosteroids. Detailed inclusion/exclusion criteria are described in Method S1.
Study treatment and other medications
Three doses of HDM tablets, that is, 100 IR, 300 IR, and 500 IR, were administered to the patients in the active treatment groups. Tablets of 100 IR were administered for the dose‐escalation period and then two dose levels of HDM tablets (300 IR and 500 IR) were administered to the respective active groups. The active tablets contained a 1 : 1 mixture of standardized extracts of D. pteronyssinus and D. farinae. The dosing method is described in Method S2. The information on allergen content was published previously 6. All active and placebo tablets were supplied by Stallergenes S.A. (Antony, France).
Assessments
Patients assessed their four nasal symptoms (0–4 for sneezing, rhinorrhea, and nasal congestion; and 0–3 for nasal pruritus), two ocular symptoms (0–4 for itchy and watering eyes), troubles with daily life (0–4), and rescue medication use on a daily basis during each two‐week interim evaluation period (weeks 8–10, 16–18, 24–26, 32–34, and 40–42) and the primary evaluation period (weeks 44–52, i.e., the last eight weeks of the treatment). Scoring of symptoms was performed based on the Practical Guideline for the Management of AR in Japan 9, 10, with modifications (Methods S3–S5). A medication score of 1 was assigned if a subject took an oral and/or ocular antihistamine, and a score 2 was assigned if a subject took a nasal corticosteroid (regardless of antihistamine use). An intranasal examination was performed by the investigators (Method S6). The patients also assessed their quality of life (QOL) by filling out the Japanese Rhinoconjunctivitis Quality of Life Questionnaire (JRQLQ) 9, 11 and evaluated the global treatment efficacy by comparing their symptoms before and after the treatment. Serum IgE and IgG4 specific to D. pteronyssinus and D. farinae and total IgE levels were determined (BML Inc., Kawagoe, Japan) at baseline and week 52.
Outcomes
The primary efficacy endpoint was the Average Adjusted Symptom Score (AASS) in the last eight weeks of the 52‐week treatment period 12. For each patient, the AASS was the average of the Adjusted Symptom Scores (ASSs) over the primary evaluation period. The ASS is based on ‘the highest observation carried forward basis’ approach and was calculated for each patient as follows. If a patient did not take rescue medication on the day of scoring or the day prior to that, the ASS was equal to the Rhinitis Total Symptom Score (RTSS). If a patient took rescue medication on the day of scoring, the ASS was equal to the day's RTSS or the ASS of the previous day, whichever was higher. The ASS on the next day of rescue medication use was equal to the RTSS on the next day or the ASS from the prior day, whichever was higher. The RTSS (range: 0–15) was the sum of the four individual rhinitis symptom scores. The Total Rhinoconjunctivitis Symptom Score was the sum of the four nasal and two ocular individual symptom scores. A Combined Score (range: 0 to 2.875) was calculated as follows: (RTSS/4 + medication score)/2.
Data sets analyzed
The full analysis set (FAS) was defined as all randomized patients, excluding those with GCP noncompliance, those receiving no study drug, and those without any efficacy data. The safety analysis set was defined as those patients receiving study drugs at least once.
Statistical analyses
The primary efficacy endpoint was the AASS assessed during the last eight weeks of the treatment period in the FAS. The differences in the AASS during the last eight weeks were assessed between each of the active groups and the placebo group using a mixed‐effects model for repeated measures (MMRM) approach. The detailed statistical analysis method is described in Method S7.
Results
Study population
A total of 968 patients were randomized to the 300 IR (n = 322), 500 IR (n = 323), and placebo (n = 323) treatment groups. Of them, 853 (88.1%) completed the study. The FAS was comprised of 927 patients (300 IR: n = 315; 500 IR: n = 296; placebo: n = 316). Compared to the other groups, a larger proportion of patients in the 500 IR group had adverse events (AEs), leading to withdrawal before the first diary entries. These patients were excluded from the FAS. The demographic profiles were similar in all three treatment groups (Table 1).
Table 1.
Demographics and baseline characteristics (FAS)
| 300 IR (n = 315) | 500 IR (n = 296) | Placebo (n = 316) | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age (years) | Mean ± SD | 30.0 ± 11.8 | 30.5 ± 11.7 | 30.2 ± 11.6 |
| 12≤ to <18 | 57 (18.1) | 55 (18.6) | 59 (18.7) | |
| 18≤ to <51 | 245 (77.8) | 228 (77.0) | 245 (77.5) | |
| 51≤ to <65 | 13 (4.1) | 13 (4.4) | 12 (3.8) | |
| Gender | Male | 145 (46.0) | 126 (42.6) | 137 (43.4) |
| Female | 170 (54.0) | 170 (57.4) | 179 (56.6) | |
| Rescue medication use during pretreatment period | Yes | 23 (7.3) | 18 (6.1) | 23 (7.3) |
| No | 292 (92.7) | 278 (93.9) | 293 (92.7) | |
| Duration of perennial allergic rhinitis | <5 years | 19 (6.0) | 20 (6.8) | 20 (6.3) |
| 5≤ to <10 years | 75 (23.8) | 66 (22.3) | 62 (19.6) | |
| 10 years ≤ | 221 (70.2) | 210 (70.9) | 234 (74.1) | |
| Serum‐specific IgE level to Dermatophagoides pteronyssinus (Ua/ml) | <0.35 | 0 | 0 | 1 (0.3) |
| 0.35≤ to <0.70 | 0 | 1 (0.3) | 1 (0.3) | |
| 0.70≤ to <3.5 | 51 (16.2) | 48 (16.2) | 42 (13.3) | |
| 3.5≤ to <17.5 | 102 (32.4) | 107 (36.1) | 125 (39.6) | |
| 17.5≤ to <50 | 91 (28.9) | 77 (26.0) | 79 (25.0) | |
| 50≤ to <100 | 45 (14.3) | 42 (14.2) | 51 (16.1) | |
| 100≤ | 26 (8.3) | 21 (7.1) | 17 (5.4) | |
| Serum‐specific IgE level to D. farinae (Ua/ml) | <0.35 | 0 | 0 | 1 (0.3) |
| 0.35≤ to <0.70 | 5 (1.6) | 2 (0.7) | 0 | |
| 0.70≤ to <3.5 | 38 (12.1) | 43 (14.5) | 37 (11.7) | |
| 3.5≤ to <17.5 | 105 (33.3) | 97 (32.8) | 110 (34.8) | |
| 17.5≤ to <50 | 86 (27.3) | 89 (30.1) | 98 (31.0) | |
| 50≤ to <100 | 57 (18.1) | 38 (12.8) | 54 (17.1) | |
| 100≤ | 24 (7.6) | 27 (9.1) | 16 (5.1) | |
| Sensitization status | Monosensitizeda | 99 (31.4) | 88 (29.7) | 98 (31.0) |
| Polysensitizedb | 216 (68.6) | 208 (70.3) | 218 (69.0) | |
| Average Rhinitis Total Symptom Score at baseline | Mean ± SD | 9.05 ± 2.03 | 9.00 ± 1.93 | 9.06 ± 2.01 |
| <10 | 220 (69.8) | 208 (70.3) | 220 (69.6) | |
| 10≤ to <13 | 79 (25.1) | 75 (25.3) | 80 (25.3) | |
| 13≤ | 16 (5.1) | 13 (4.4) | 16 (5.1) | |
No sensitization other than D. pteronyssinus or D. farinae among the tested allergens (level of serum‐specific IgE against all other allergens less than 0.70 Ua/ml).
At least one sensitization in addition to D. pteronyssinus or D. farinae (level of serum‐specific IgE against any other allergen is 0.70 Ua/ml or higher).
Efficacy outcomes
The AASSs over week 44–52 (primary endpoint) in both the 300 and 500 IR groups significantly improved compared to that in the placebo group (P < 0.001). The mean absolute and relative differences of the AASS (vs placebo) were −1.11 (−18.2%) for the 300 IR group and −0.80 (−13.1%) for the 500 IR group (Table 2). The difference between the 300 IR and 500 IR groups was not statistically significant. Compared to placebo, the AASS in the 300 IR group was significantly improved at week 8–10 (P = 0.0012). This improvement was maintained throughout the treatment period, which indicates that the onset of action was at week 8–10 (Fig. 1). A statistically significant improvement in the AASS was also observed in the 500 IR group at week 8–10 (P = 0.0448), which was maintained throughout the treatment period with the exception of week 16–18. Similarly, the ARTSS and average Combined Score at week 44–52 consistently and significantly improved in both active treatment groups compared to those in placebo (P < 0.001). Furthermore, both active treatment groups showed a statistically significant improvement in the Average Total Rhinoconjunctivitis Symptom Score and all four individual nasal symptom scores compared to those in the placebo group. The ocular symptom scores were generally modest, 1.0 or less in the placebo group. The watering eye score improved only in the 300 IR group, while there was no significant difference in itchy eye in either active treatment group vs placebo (Table 2). Although rescue medication use was limited, and the medication score was as low as 0.07 in the placebo group, the 300 IR group showed a statistically significant reduction in these measures vs placebo (P = 0.0280). The troubles with daily life score was significantly improved in both active treatment groups. Observed mean ± standard deviation (SD) values at baseline and week 44–52 and the changes are shown in Table S1.
Table 2.
Symptom and medication scores at week 44–52, primary evaluation period (FAS)
| Score | 300 IR (n = 315) | 500 IR (n = 296) | Placebo (n = 316) |
|---|---|---|---|
| Average Adjusted Symptom Score (AASS, primary variable) | 5.00 ± 0.21*** | 5.32 ± 0.22*** | 6.11 ± 0.21 |
| Difference from. Placebo | |||
| Least squares mean difference | −1.11 ± 0.20*** | −0.80 ± 0.20*** | |
| Relative least squares mean difference | −18.2% | −13.1% | |
| Average Rhinitis Total Symptom Score (ARTSS) | 4.96 ± 0.21*** | 5.25 ± 0.21*** | 6.03 ± 0.21 |
| Average Medication Score | 0.04 ± 0.02* | 0.07 ± 0.02 | 0.07 ± 0.02 |
| Average Combined Score | 0.62 ± 0.03*** | 0.67 ± 0.03*** | 0.77 ± 0.03 |
| Average Total Rhinoconjunctivitis Symptom Score | 6.48 ± 0.29*** | 6.91 ± 0.30** | 7.79 ± 0.29 |
| Individual nasal and ocular symptom scores | |||
| Sneezing | 1.13 ± 0.05** | 1.14 ± 0.05** | 1.27 ± 0.05 |
| Rhinorrhea | 1.43 ± 0.07*** | 1.52 ± 0.07*** | 1.74 ± 0.07 |
| Nasal congestion | 1.22 ± 0.07*** | 1.35 ± 0.07*** | 1.58 ± 0.07 |
| Nasal pruritus | 1.18 ± 0.06*** | 1.23 ± 0.06*** | 1.43 ± 0.06 |
| Itchy eyes | 0.92 ± 0.06 | 0.97 ± 0.06 | 1.03 ± 0.06 |
| Watering eyes | 0.59 ± 0.05* | 0.68 ± 0.06 | 0.72 ± 0.05 |
| Troubles with daily life | 1.02 ± 0.06*** | 1.11 ± 0.06** | 1.28 ± 0.06 |
The data are presented as the least squares mean ± standard error.
The relative least squares mean difference: {(Active − Placebo)/Placebo} × 100.
The mixed‐effects model for repeated measures (MMRM) includes the terms for the treatment group, time, and treatment‐by‐time as fixed effects; and the baseline value, age, gender, sensitization status with autumn allergies, rescue medication use during the pretreatment period, and prior drug for the target disease as covariates.
***P < 0.001, **P < 0.01, *P < 0.05 compared with placebo.
Figure 1.
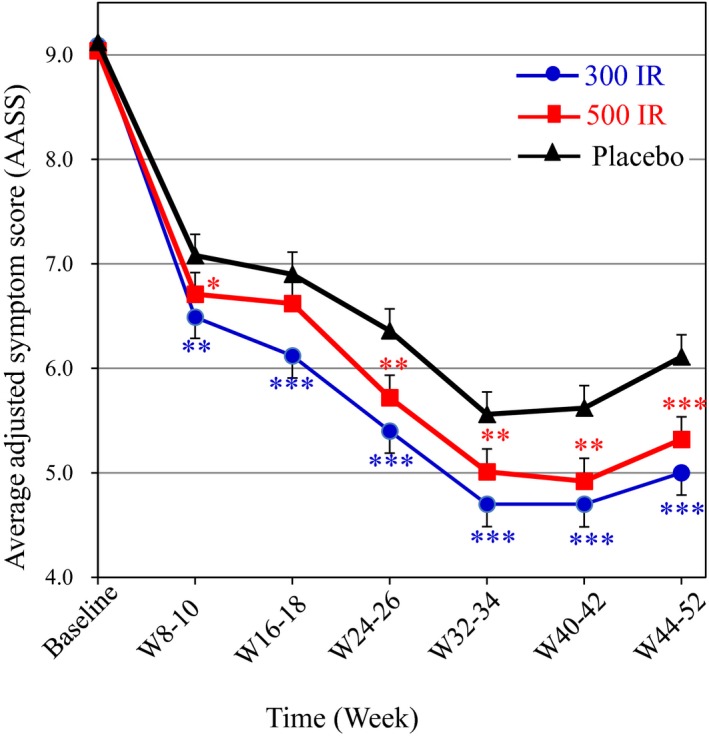
Time course of the Average Adjusted Symptom Score (AASS) for the 52‐week treatment period (FAS). The least squares means and standard errors with the mixed‐effects model for repeated measures (MMRM) are shown for the time points at week 8–10 and later. The means of the observed values are shown for the baseline. ***P < 0.001, **P < 0.01, *P < 0.05 compared with placebo.
In the JRQLQ, all of the three main domains, that is, the nasal eye symptom scores, QOL‐related questionnaire scores, and general state, significantly improved in the 300 IR group compared to those in the placebo group (Table 3). Regarding scores from the QOL‐related questionnaire, four (usual daily activities, outdoor activities, social functioning, and physical problems) of six subdomains were significantly improved in the 300 IR group compared to the placebo group. In the 500 IR group, all main and subdomains showed a trend of improvement but did not reach statistical significance. Observed values (means ± SD) at baseline and week 52 and the changes are shown in Table S2.
Table 3.
JRQLQ scores at 52‐week treatment (FAS)
| Score | 300 IR (n = 315) | 500 IR (n = 296) | Placebo (n = 316) |
|---|---|---|---|
| Nasal and eye symptoms | 7.17 ± 0.34* | 7.58 ± 0.34 | 8.00 ± 0.33 |
| QOL‐related questionnaires | 9.17 ± 0.89** | 10.45 ± 0.90 | 11.66 ± 0.88 |
| Usual daily activities | 3.37 ± 0.30** | 3.84 ± 0.31 | 4.22 ± 0.30 |
| Outdoor activities | 0.66 ± 0.11** | 0.85 ± 0.11 | 0.98 ± 0.11 |
| Social functioning | 1.16 ± 0.16** | 1.35 ± 0.16 | 1.61 ± 0.16 |
| Impaired sleeping | 0.66 ± 0.07 | 0.68 ± 0.07 | 0.77 ± 0.07 |
| Physical problems | 1.44 ± 0.14* | 1.66 ± 0.15 | 1.76 ± 0.14 |
| Emotional functions | 1.92 ± 0.23 | 2.13 ± 0.23 | 2.32 ± 0.23 |
| General state | 1.54 ± 0.07* | 1.57 ± 0.07 | 1.69 ± 0.07 |
The data are presented as the least squares mean ± standard error.
The mixed‐effects model for repeated measures (MMRM) includes the terms for the treatment group, time, and treatment‐by‐time as fixed effects; and the baseline value, age, gender, sensitization status with autumn allergies, rescue medication use during the pretreatment period, and prior drug for the target disease as covariates.
**P < 0.01, *P < 0.05 compared with placebo.
Both nasal mucosal swelling and nasal watery secretion in physicians’ intranasal examination markedly and significantly improved in both active treatment groups compared to those in the placebo group (Tables S3 and S4).
For patients’ global evaluation of treatment efficacy, the percentages of patients with slight‐to‐moderate and marked improvement in both active treatment groups were significantly higher than those in the placebo group (Table S5).
Immunological responses
The geometric means of the serum immunoglobulins at baseline and week 52 are shown in Fig. 2. The levels of IgE and immunoglobulin G4 (IgG4) specific to HDM were increased 1.8‐ to 1.9‐fold and 2.6‐ to 3.7‐fold, respectively, in the active treatment groups, while there were no marked changes in the placebo group.
Figure 2.
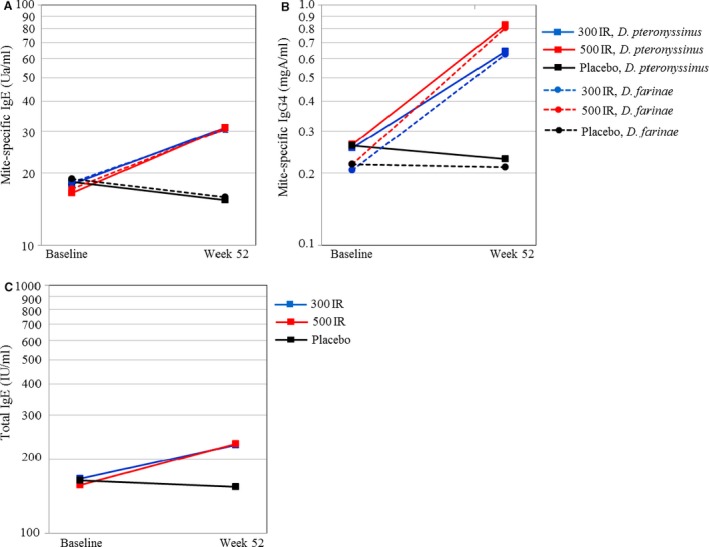
Serum immunoglobulin (Ig). Geometric means at baseline and at week 52 are shown. A. Mite‐specific IgE; B. mite‐specific IgG4; C. total IgE.
Safety outcomes
The 300 IR and 500 IR HDM tablets were generally safe and well tolerated. The reported AEs are summarized in Table 4. The overall incidences of AEs and adverse drug reactions (ADRs, AEs for which causal relationship with the study drug could not be ruled out by the investigators) were higher in the active treatment groups than in the placebo group, and the incidence of AEs leading to withdrawal was higher in the 500 IR group. AEs considered to be local allergic reactions were observed more frequently in the active treatment groups than in the placebo group. The most frequent local allergic AEs were throat irritation, edema mouth, oral pruritus, and ear pruritus. Most of these AEs were mild, and there was a trend of recovery with the study drug treatment. Sixteen serious AEs (SAEs) were reported: one case each of appendicitis, gastroenteritis, bacterial pneumonia, cholelithiasis, hematuria, and abortion observed in the 300 IR group; two cases of diverticulitis and one case each of appendicitis, gastroenteritis Escherichia coli infection, hepatitis B, inguinal hernia, large intestine polyp, and cholelithiasis in the 500 IR group; and one case each of forearm fracture and ligament injury in the placebo group. None of these SAEs were judged to be related to the study drug. No cases of anaphylactic shock were reported. There were no marked abnormalities in clinical laboratory results and vital signs in any group.
Table 4.
Incidence of adverse events
| 300 IR (n = 322) | 500 IR (n = 324) | Placebo (n = 322) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Subjects with any AEs | 284 (88.2)* | 294 (90.7)* | 243 (75.5) |
| Serious AEs | 6 (1.9) | 5 (1.5) | 2 (0.6) |
| AEs leading to withdrawal | 14 (4.3) | 29 (9.0)* | 12 (3.7) |
| Subjects with any ADRs | 215 (66.8)* | 237 (73.1)* | 60 (18.6) |
| Serious ADRs | 0 | 0 | 0 |
| ADRs leading to withdrawal | 7 (2.2)* | 21 (6.5)* | 0 |
| AEs with incidence of 5% or more in either active group | |||
| Infections and infestations | 204 (63.4) | 192 (59.3) | 201 (62.4) |
| Nasopharyngitis | 117 (36.3) | 99 (30.6) | 116 (36.0) |
| Pharyngitis | 55 (17.1) | 60 (18.5) | 58 (18.0) |
| Gastroenteritis | 20 (6.2) | 21 (6.5) | 17 (5.3) |
| Influenza | 18 (5.6) | 19 (5.9) | 19 (5.9) |
| Acute sinusitis | 18 (5.6) | 18 (5.6) | 20 (6.2) |
| Respiratory, thoracic and mediastinal disorders | 114 (35.4)* | 123 (38.0)* | 39 (12.1) |
| Throat irritation | 67 (20.8)* | 66 (20.4)* | 12 (3.7) |
| Oropharyngeal discomfort | 17 (5.3)* | 23 (7.1)* | 4 (1.2) |
| Ear and labyrinth disorders | 57 (17.7)* | 52 (16.0)* | 11 (3.4) |
| Ear pruritus | 45 (14.0)* | 44 (13.6)* | 3 (0.9) |
| Gastrointestinal disorders | 173 (53.7)* | 199 (61.4)* | 59 (18.3) |
| Edema mouth | 67 (20.8)* | 81 (25.0)* | 1 (0.3) |
| Oral pruritus | 36 (11.2)* | 51 (15.7)* | 7 (2.2) |
| Stomatitis | 28 (8.7)* | 25 (7.7)* | 12 (3.7) |
| Oral discomfort | 14 (4.3)* | 20 (6.2)* | 4 (1.2) |
The data are presented as the number and percentage (in parentheses) of patients who experienced adverse events (AEs).
ADR, adverse drug reaction.
*P < 0.05 compared with placebo, Fisher's exact test.
Discussion
This study is one of the largest (968 patients) clinical trials to evaluate the efficacy and safety of AIT. As it is recommended that the primary endpoint of an AIT study should reflect both rescue medication intake and symptoms 13, 14, we set the AASS as a primary variable, as previously described 6. A significant improvement in the AASS was observed at week 8–10, which was maintained throughout week 44–52 in the 300 IR group. The time of action onset was one of the earliest reported in an HDM AIT study based on a conventional natural exposure design.
In this study, there was a marked decrease in AASS in the placebo group, as seen in other clinical studies in which efficacy evaluation was performed in a subjective way, for example, AR trials 15, including the recent trial of another HDM SLIT product 7. One possible cause of this phenomenon is a psychological effect where subjects feel improvement of their symptoms by the action of taking a pill 16. Vits et al. reported that cognitive factors, such as patient's expectations, seemed to induce the placebo effect in AR patients 17. The present study was the first efficacy clinical trial of HDM SLIT in Japan, so the participants might have had overoptimistic expectations for this ‘novel’ therapy.
Another reason for the improvements seen in the placebo group may be the fluctuation in AR symptoms 15. In this study, patients were required to have an ARTSS of 6 or higher to be enrolled, and the actual mean baseline ARTSS was as high as 9, in which subjects’ disease state may be aggravated. After entering the study, the severity of their symptoms may have decreased toward their ordinary level, so‐called regression to the mean 18. Seasonal fluctuation of symptoms could also have contributed to the effects in the placebo group, as AR symptoms by mite allergen have been reported to be aggravated in autumn 19, 20. In this study, baseline evaluation (for 7 days just before randomization) was performed between October 18 and December 2, 2012, and the primary efficacy evaluation (week 44–52) was performed between August 23 and December 6, 2013. Both assessments were in autumn. Time profile of AR symptoms in this study may partially reflect seasonal fluctuation: with high AR symptoms at baseline, markedly declined symptoms until week 32–34, and increased symptoms again at week 44–52 in both treatment and placebo groups.
In a recent study of another HDM SLIT product 7, rescue medication use was not restricted, and its use may have caused the decrease in AR symptoms. In the present study, however, rescue medication use was allowed only when the symptoms were intolerable or interfered with daily activities to evaluate the AR symptoms correctly. Actual use of the rescue medication was small, and, therefore, it was unlikely that the marked decrease in AASS was caused by rescue medication use.
It has been proposed by the World Allergy Organization (WAO) taskforce that a clinically relevant difference between active and placebo treatments should be 20% or greater 13. Although the relative difference did not reach the 20% threshold in the primary endpoint in this study, we speculate that a marked decrease in the placebo treatment group interfered with the accurate evaluation of the outcome and masked the effect of the active treatment. In other clinical studies of perennial AR with a marked decrease in the symptom score in the placebo group, the difference in the symptoms between active and placebo treatment groups became close 21, 22, 23. The added benefits afforded by the 300 IR HDM tablet were supported by consistent and significant improvements in other endpoints, for example, all four individual nasal symptom scores, the medication score, the combined score, the intranasal examination score, the global treatment efficacy assessment, and all of the three main domains of JRQLQ and four subdomains in QOL‐related questionnaires. The significant improvement in QOL is particularly relevant for assessing clinical efficacy because the assessment of QOL is recommended by the WAO taskforce for AIT study 13, and it reflects the systemic burden of allergic disease, which cannot be assessed by symptom score. In addition, in several efficacy endpoints, including the primary endpoint, the P‐values were as low as <0.001 when comparing the 300 IR and the placebo groups. Therefore, a clear clinical effect of 300 IR HDM tablet was identified in this study.
We found no statistical difference between 300 IR and 500 IR in the AASS at week 44–52, a primary endpoint, while a slightly better trend was seen with 300 IR than 500 IR. It is known that AIT induces not only tolerogenic effects but also adverse allergic reactions by administered allergens. In this study, the incidence of ADRs and ADRs leading to withdrawal tended to be higher at 500 IR than at 300 IR. These findings suggest that administering 500 IR could be over the optimal allergen quantity and affected the subjects’ QOL and the symptom score. The effect of 500 IR, however, was found to be comparable or favorable to that of 300 IR in a previous European study 6. Moreover, a recent environmental exposure chamber study showed that HDM tablets at 100 IR, 300 IR, and 500 IR taken for six months in patients with AR dose‐dependently improved symptoms. The 500 IR dose was associated with the greatest reduction in allergen‐induced symptoms but showed no statistically significant difference vs 300 IR 24. Taken together, these data demonstrate that there is no relevant difference in efficacy between 300 IR and 500 IR.
This study was not designed to evaluate the cost‐effectiveness of HDM SLIT tablet prospectively. For other AITs, the cost‐effectiveness of grass SLIT tablet was prospectively evaluated in a placebo‐controlled pivotal study, and a favorable pharmacoeconomic effect was shown 25. The HDM is a perennial allergen, and most patients who suffer from HDM‐induced AR exhibit symptoms year‐round, in contrast to the limited duration of the seasonal grass pollen allergy. In addition, in the previous HDM tablet study, a carryover effect was demonstrated after a 1‐year AIT‐free period 6. These points may favorably affect the cost‐effectiveness, although further evaluation is necessary.
The levels of HDM‐specific IgG4 increased to similar levels as those reported previously 6. A modest increase in HDM‐specific IgE and total IgE was also observed in the active treatment groups at week 52. As specific IgE has been reported to increase at the early stage of AIT and decrease thereafter 26, we estimated that levels of specific and total IgE are still elevated at the 1‐year time point.
Regarding the safety profile, local reactions typical of SLIT were observed, but the majority of them were mild in severity. The types and incidence of AEs were similar to those observed in a previous study 6.
In conclusion, the 300 IR and 500 IR HDM tablets were effective and safe during a 52‐week treatment period in patients with AR. The onset of action was at week 8–10 in the 300 IR group, and we recommend a therapeutic dose of 300 IR for AR. Overall profiles of efficacy, safety, and immunological response are similar to those in the previous European study.
Author contributions
Yoshitaka Okamoto, Shigeharu Fujieda, Mitsuhiro Okano, Shinji Kakudo, and Keisuke Masuyama substantially contributed to the conception and design of the study, acquisition of data, and interpretation of data. Yuki Yoshida was responsible for statistical analysis. All authors approved the final manuscript.
Conflict of interest
This study was funded by Shionogi. Yoshitaka Okamoto, Shigeharu Fujieda, Mitsuhiro Okano, and Keisuke Masuyama received a consultant fee from Shionogi. Yuki Yoshida and Shinji Kakudo are employees of Shionogi.
Supporting information
Method S1. Study participants.
Method S2. Study treatment and other medications.
Method S3. Severity scoring of nasal symptoms.
Method S4. Severity scoring of eye symptoms.
Method S5. Severity of troubles with daily life.
Method S6. Intranasal examination by investigators.
Method S7. Statistical analysis.
Table S1. Observed values (mean ± SD) of symptom and medication scores at baseline and week 44–52.
Table S2. Observed values (mean ± SD) of JRQLQ scores at baseline and week 52.
Table S3. Intranasal examination at Week 52.
Table S4. Observed values (mean ± SD) of intranasal examination at baseline and week 52.
Table S5. Global evaluation of treatment efficacy by subjects at Week 52 (FAS).
Data S1. Study investigators.
Acknowledgments
The authors would like to thank all the investigators (Data S1).
Okamoto Y, Fujieda S, Okano M, Yoshida Y, Kakudo S, Masuyama K. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy 2017; 72:435–443.
Edited by: Pascal Demoly
References
- 1. Calderón MA, Linneberg A, Kleine‐Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol 2015;13:38–48. [DOI] [PubMed] [Google Scholar]
- 2. Calderón MA, Carr VA, Jacobson M, Sheikh A, Durham S. Allergen injection immunotherapy for perennial allergic rhinitis. Cochrane Database Syst Rev 2008;2:CD007163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radulovic S, Calderón MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010;12:CD002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence‐based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol 2013;132:1322–1336. [DOI] [PubMed] [Google Scholar]
- 5. Calderón MA, Kleine‐Tebbe J, Linneberg A, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC. House dust mite respiratory allergy: an overview of current therapeutic strategies. J Allergy Clin Immunol Pract 2015;3:843–855. [DOI] [PubMed] [Google Scholar]
- 6. Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol 2014;133:1608–1614. [DOI] [PubMed] [Google Scholar]
- 7. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine‐Tebbe J. Effective treatment of house dust mite‐induced allergic rhinitis with 2 doses of the SQ HDM SLIT‐tablet: results from a randomized double‐blind, placebo‐controlled phase III trial. J Allergy Clin Immunol 2016;137:444–451. [DOI] [PubMed] [Google Scholar]
- 8. Pastorello EA, Incorvaia C, Ortolani C, Bonini S, Canonica GW, Romagnani S et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol 1995;96:580–587. [DOI] [PubMed] [Google Scholar]
- 9. Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H et al. Japanese Society of Allergology. Japanese guideline for allergic rhinitis 2014. Allergol Int 2014;63:357–375. [DOI] [PubMed] [Google Scholar]
- 10. Fujieda S, Kurono Y, Okubo K, Ichimura K, Enomoto T, Kawauchi H et al. Examination, diagnosis and classification for Japanese allergic rhinitis: Japanese guideline. Auris Nasus Larynx 2012;39:553–556. [DOI] [PubMed] [Google Scholar]
- 11. Okubo K, Gotoh M, Fujieda S, Okano M, Yoshida H, Morikawa H et al. A randomized double‐blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol Int 2008;57:265–275. [DOI] [PubMed] [Google Scholar]
- 12. Grouin JM, Vicaut E, Jean‐Alphonse S, Demoly P, Wahn U, Didier A et al. The average Adjusted Symptom Score, a new primary efficacy end‐point for specific allergen immunotherapy trials. Clin Exp Allergy 2011;41:1282–1288. [DOI] [PubMed] [Google Scholar]
- 13. Canonica GW, Baena‐Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy 2007;62:317–324. [DOI] [PubMed] [Google Scholar]
- 14. Committee for Medicinal Products for Human Use . Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. CHMP/EWP/18504/2006 London: European Medicines Agency; 2008. [Google Scholar]
- 15. del Cuvillo A, Sastre J, Bartra J, Mullol J, DáVila I, Montoro J et al. Placebo effect in clinical trials involving patients with allergic rhinitis. J Investig Allergol Clin Immunol 2011;21(Suppl 3):40–45. [PubMed] [Google Scholar]
- 16. Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci 2005;25:10390–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vits S, Cesko E, Benson S, Rueckert A, Hillen U, Schadendorf D et al. Cognitive factors mediate placebo responses in patients with house dust mite allergy. PLoS One 2013;18:e79576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barnett AG, van der Pols JC, Dobson AJ, Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 19. Miyazawa H, Sakaguchi M, Inouye S, Ikeda K, Honbo Y, Yasueda H et al. Seasonal changes in mite allergen (Der I and Der II) concentrations in Japanese homes. Ann Allergy Asthma Immunol 1996;76:170–174. [DOI] [PubMed] [Google Scholar]
- 20. Pfaar O, Gerth van Wijk R. Mite‐allergic rhinitis: how to evaluate clinical efficacy in allergen‐specific immunotherapy trials? Curr Treat Options Allergy 2015;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frèche C, Leynadier F, Horak F, Hide D, Gracia FD, Goos M et al. Mizolastine provides effective symptom relief in patients suffering from perennial allergic rhinitis: a double‐blind, placebo‐controlled study versus loratadine. Ann Allergy Asthma Immunol 2002;89:304–310. [DOI] [PubMed] [Google Scholar]
- 22. Bousquet J, Bachert C, Canonica GW, Mullol J, Van Cauwenberge P, Jensen CB et al. Efficacy of desloratadine in persistent allergic rhinitis – a GA²LEN study. Int Arch Allergy Immunol 2010;153:395–402. [DOI] [PubMed] [Google Scholar]
- 23. Potter PC, Paediatric Levocetirizine Study Group . Efficacy and safety of levocetirizine on symptoms and health‐related quality of life of children with perennial allergic rhinitis: a double‐blind, placebo‐controlled randomized clinical trial. Ann Allergy Asthma Immunol 2005;95:175–180. [DOI] [PubMed] [Google Scholar]
- 24. Roux M, Devillier P, Yang WH, Montagut A, Abiteboul K, Viatte A et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: results of a dose‐ranging study in an environmental exposure chamber. J Allergy Clin Immunol 2016;138:451–458. [DOI] [PubMed] [Google Scholar]
- 25. Bachert C, Vestenbaek U, Christensen J, Griffiths UK, Poulsen PB. Cost‐effectiveness of grass allergen tablet (GRAZAX) for the prevention of seasonal grass pollen induced rhinoconjunctivitis – a Northern European perspective. Clin Exp Allergy 2007;37:772–779. [DOI] [PubMed] [Google Scholar]
- 26. Akdis M, Akdis CA. Mechanisms of allergen‐specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol 2014;133:621–631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Method S1. Study participants.
Method S2. Study treatment and other medications.
Method S3. Severity scoring of nasal symptoms.
Method S4. Severity scoring of eye symptoms.
Method S5. Severity of troubles with daily life.
Method S6. Intranasal examination by investigators.
Method S7. Statistical analysis.
Table S1. Observed values (mean ± SD) of symptom and medication scores at baseline and week 44–52.
Table S2. Observed values (mean ± SD) of JRQLQ scores at baseline and week 52.
Table S3. Intranasal examination at Week 52.
Table S4. Observed values (mean ± SD) of intranasal examination at baseline and week 52.
Table S5. Global evaluation of treatment efficacy by subjects at Week 52 (FAS).
Data S1. Study investigators.


