Figure 3.
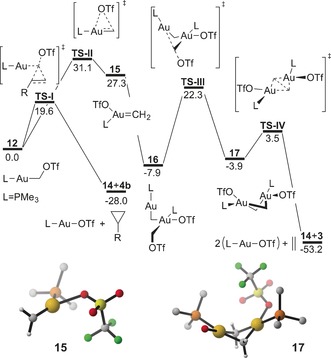
Calculated reaction profiles for the cyclopropanation of norbornene and formation of ethylene on the model system. DFT calculations were performed at B3LYP‐D3/6‐31G(d,p)+SDD(f,g) on Au. Toluene was represented with the PCM. Free energies are in kcal mol−1. Optimized structure of the proposed intermediates 15 and 17 are shown. Non‐CH2 H atoms as well as front facing TfO− in 17 are omitted for clarity. Selected bond distances [Å] and angles [°] for 15: Au–CH2 1.906, Au–O 2.281,30 Au–P 2.403; H2C‐Au‐O 144.2, H2C‐Au‐P 139.7, O‐Au‐P 76.1; and 17: Au–CH2 2.079, Au–Au 2.983, CH2–CH2 2.461, Au–O 2.222, Au–P 2.406; H2C‐Au‐O 100.5, H2C‐Au‐P 98.4, O‐Au‐P 87.9.
