Figure 2.
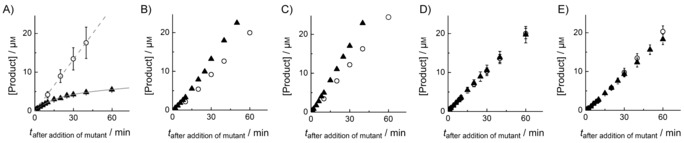
FGE4C‐catalyzed product formation as a function of time (○) in reactions containing 0.5 μm FGE4C, 200 μm substrate, 0.5 μm CuSO4, 2 mm DTT, 50 mm EDTA, 50 mm NaCl and 50 mm Tris (pH 8). The reaction mixtures were supplemented with 5 μm A) FGES266A, B) FGEC274S, C) FGEC269S, D) BSA, or E) FGES290K, one minute after initiation with FGE4C (▴). In A), ▴ data were fitted to the function [P]=A(1−exp(−k t))+m 1 t (—), and the ○ data were fitted to [P]=m 2 t (‐ ‐ ‐ ‐), where A is the concentration of product formed until CuI redistribution between FGE4C and FGES266A is complete (4.0 μm), k is the rate at which the FGE4C:CuI complex decays (0.07 min−1), m 1 is the residual activity after CuI redistribution (m 1/[FGE4C]=0.05 min−1), and m 2 is the activity of CuI‐complemented FGE4C (m 2/[FGE4C]=0.8 min−1). The data are averaged values from two or more independent experiments.
