Figure 3.
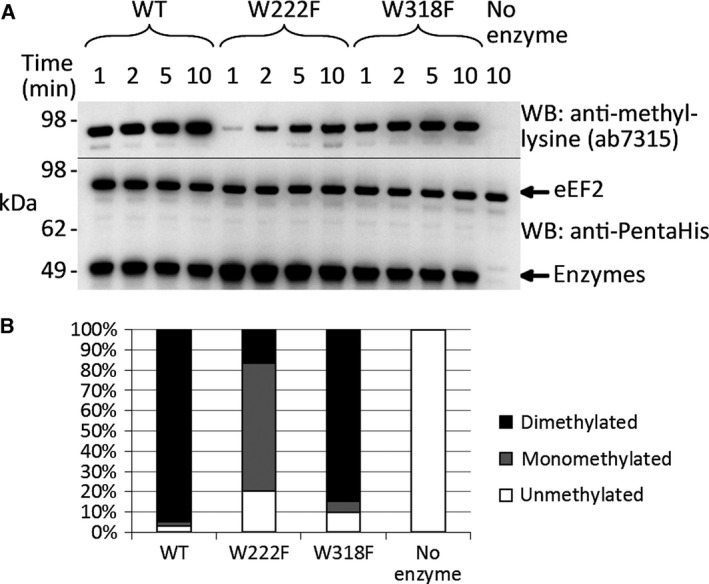
Tryptophan point mutants of Efm2 show reduced methyltransferase activity. eEF2 was incubated with wild‐type (WT), W222F or W318F Efm2 in the presence of AdoMet for 1, 2, 5 or 10 min, before reactions were resolved by SDS/PAGE and analysed by immunoblotting (A) or LC‐MS/MS (B). No enzyme was added for a negative control. (A) Immunoblotting with the anti‐methyl‐lysine antibody ab7315 (top) revealed markedly reduced methyltransferase activity of the W222F mutant, while the W318F mutant showed slightly reduced activity. An immunoblot with the anti‐PentaHis antibody was used as a loading control (bottom). (B) LC‐MS/MS of eEF2 from the 10‐min assay revealed that the wild‐type enzyme was able to produce almost complete dimethylation of K613 in the peptide DDFKAR, while the W222F mutant produced predominantly monomethylation and the W318F mutant produced nearly the same level of dimethylation as the wild‐type enzyme. The no enzyme control did not show any gain of methylation. The relative amount of each methylation state was determined by the area under the curve for each peak in the extracted ion chromatograms (XICs), which are shown in Fig. S4. W222 therefore appears to be important for methyltransferase activity, while W318 is not as important.
