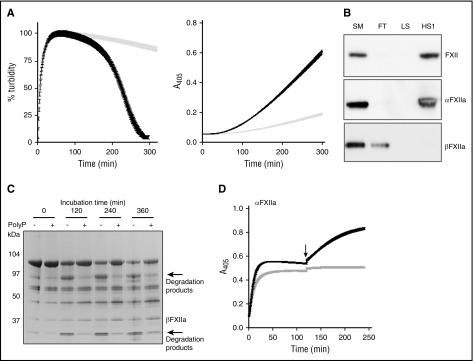
Figure 2.
PolyP70 stimulates FXII activation and modulates its plasminogen activator function. (A) PolyP induces autoactivation of FXII. Left: Clots were formed with 3.8 μM fibrinogen, 0.24 μM Glu-plasminogen, and 200 nM FXII in the absence (gray) or presence (black) of 140 μM polyP70. Clotting was initiated with 0.25 U/mL of thrombin and 10 mM CaCl2, and subsequent lysis was monitored at 405 nm. Mean data ± SEM are expressed as percentage turbidity (n = 3; P < .0001). Right: FXII (200 nM) and Glu-plasminogen (200 nM) were incubated in the absence (gray) or presence (black) of 140 μM polyP70, and plasmin activity was detected using S2251. Data represent mean ± SEM (n = 3; P < .0001). (B) PolyP binds to FXII and αFXIIa. FXII, αFXIIa, or βFXIIa (5 μg) were run through columns containing Sepabeads coated with polyP70 before collecting the flow-through fraction (FT), low-salt wash (LS; 50 mM NaCl), and high-salt wash (HS1; 1 M NaCl) and comparing with starting material (SM). Protein was detected by western blotting with an antibody to FXII. Image is representative of 3 separate experiments. (C) PolyP protects αFXIIa from autodegradation. αFXIIa (5 μg) was incubated with or without polyP70 (100 μg) before resolving on 4% to 12% Bis-Tris gels under nonreducing conditions. Data shown are representative of 3 separate experiments. (D) PolyP preserves αFXIIa activity. αFXIIa (50 nM) activity was analyzed using S2302 in the absence (gray) or presence (black) of 70 μM polyP70. After 2 hours, additional S2302 substrate was added to the reaction (arrow) and readings continued for a further 2 hours. Data represent mean ± SEM (n = 3).