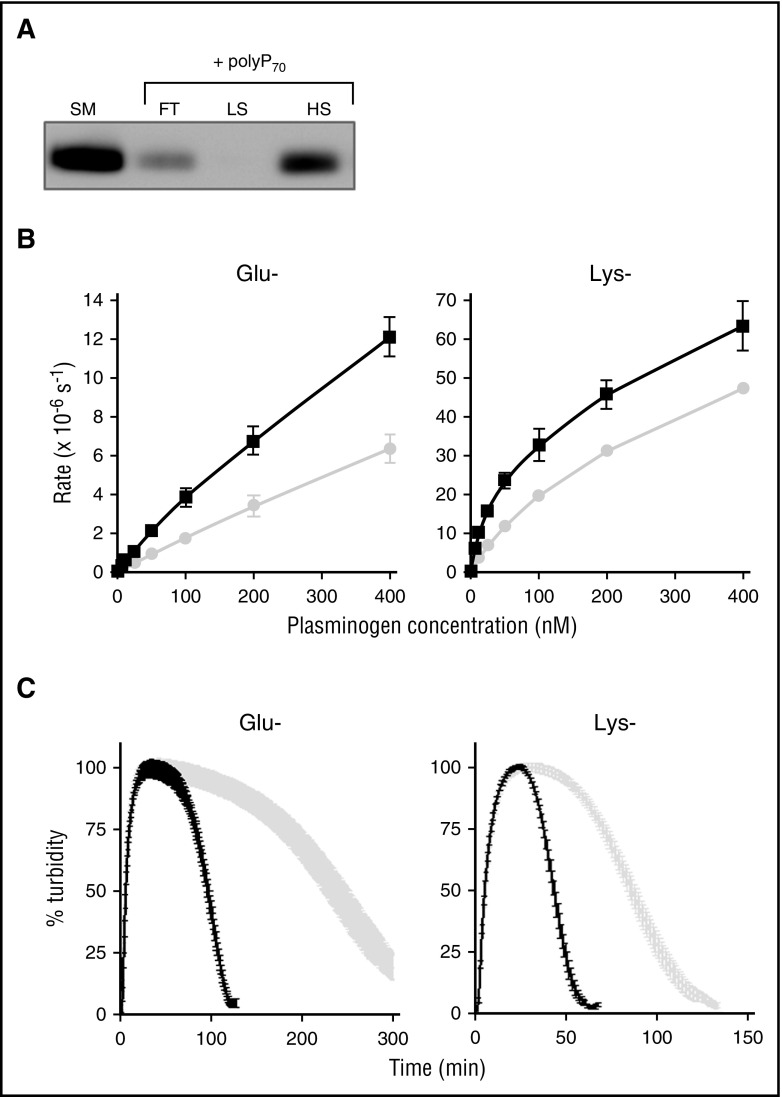
Figure 3.
αFXIIa enhances activation of Glu and Lys forms of plasminogen. (A) Binding of polyP70 plasminogen was analyzed by running Glu-plasminogen through columns containing Sepabeads coated with polyP70 before collecting the flow-through fraction (FT), low-salt wash (LS; 50 mM NaCl), and high-salt wash (HS; 1 M NaCl) and comparing with starting material (SM). Protein was detected by western blotting with an antibody to plasminogen. Image is representative of 3 separate experiments. (B) The rate of plasmin generation by αFXIIa (200 nM) in the presence (black line) or absence (gray line) of polyP70 (70 μM) was quantified for Glu-plasminogen (left; P < .05) and Lys-plasminogen (right; P < .01). Data are expressed as mean ± standard deviation (n = 3). (C) Fibrin clots were formed with fibrinogen (3.8 μM), αFXIIa (200 nM), and Glu-plasminogen (left) or Lys-plasminogen (right), in the absence (gray) or presence (black) of polyP70 (70 μM). Clotting was initiated with thrombin (0.25 U/mL) and CaCl2 (10 mM), and lysis was monitored at 405 nm. Mean data ± SEM are expressed as percentage turbidity (n = 3; P < .0001). Note the different scales on the Lys-plasminogen plot compared with Glu-plasminogen, due to the different rates of activation of the isoforms of plasminogen.