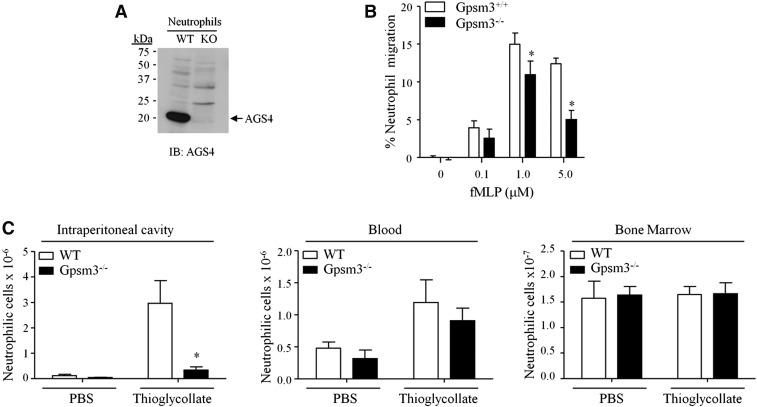
Fig. 5.
AGS4-KO neutrophils demonstrate reduced migration to site of inflammation. (A,B) Neutrophils were isolated from freshly harvested bone marrow from WT and AGS4/Gpsm3-null mice using Percoll gradient centrifugation as described in Materials and Methods. (A) Neutrophil lysates (100 μg) were prepared with 1% NP-40 lysis buffer and subjected to SDS-PAGE, transferred to PVDF, and immunoblotted with AGS4 antisera as described in Materials and Methods. The representative immunoblot shown is reflective of at least three independent experiments. (B) Isolated neutrophils were loaded in transwell migration chambers with the bottom chamber containing serum-free RPMI in the absence and presence of 0.1, 1.0, and 5.0 μM fMLP. After 3 hours at 37°C, cells in the bottom chamber were counted, and the percentage of cells migrated was calculated relative to the input where the number of cells migrating to vehicle only was subtracted. (C) WT and AGS4/Gpsm3-null mice received 1 ml of i.p. injections of 4% thioglycollate or sterile PBS to induce localized inflammation as described in Materials and Methods. Two hours postinjection, mice were euthanized and the i.p. cavity was lavaged with 10 ml of cold, sterile PBS (left panel). Blood (middle panel) was collected by cardiac stick, and femurs were processed to harvest bone marrow cells (right panel) as described in Materials and Methods. Red blood cells were lysed from cell preparations, and the remaining cells were stained with CD11b–FITC and Ly6G–PE for flow cytometry analysis of neutrophil numbers in each tissue. Neutrophil cell numbers were calculated using total events collected, applying flow rate and percentage of dual positive cells, followed by dilutions carried out during processing of the cells. Data are represented as the mean ±S.E. of n = 4 mice per genotype. *P < 0.05.